Telangana TSBIE TS Inter 1st Year Chemistry Study Material 13th Lesson Organic Chemistry: Some Basic Principles and Techniques Textbook Questions and Answers.
TS Inter 1st Year Chemistry Study Material 13th Lesson Organic Chemistry: Some Basic Principles and Techniques
Very Short Answer Type Questions
Question 1.
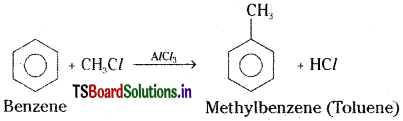
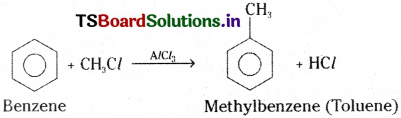
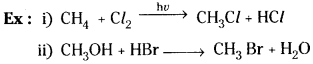
Write the reagents required for the conversion of benzene to methyl benzene.
Answer:
Benzene can be converted to methyl benzene by using methyl chloride in the presence of an hydrous AlCl3 catalyst.
![]()
Question 2.
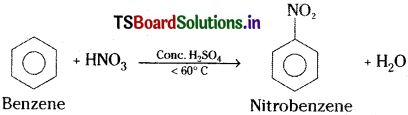
How is nitrobenzene prepared?
Answer:
When benzene is heated with a mixture of cone. HNO3 and cone. H2SO4 below 60°C, one H – atom of benzene.is substituted by NO2 groups and forms nitrobenzene.
Question 3.
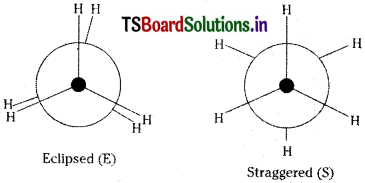
Write the conformations of ethane.
Answer:
Conformational isomerism :
As a result of rotation about C – C single bond, the atoms or groups of a molecule assume different spatial orientations. These different atomic arrangements are called conformational isomers’.
Ex: Eclipsed (E) and staggered (S) con- formers of Ethane (C2H6)
Question 4.
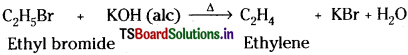
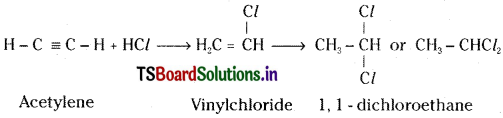
How do you prepare ethyl chloride from ethylene?
Answer:
Ethylene react with HC/ forming ethyl chloride.
CH2 = CH2 + HCl → CH3CH2Cl
![]()
Question 5.
Write the IUPAC names of: rnareum [AP. TS ’16]
a) CH3 – CH2 – CH2 – CH = CH2
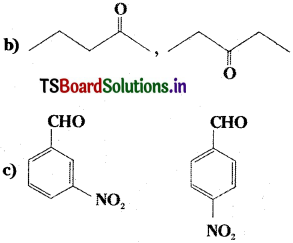
Answer:
a) 1 – pentene
b) pentane – 2 – one, pentane – 3 – one
c) 3 – nitrobenzaldehyde, 4 – nitrobenzal- dehyde.
Question 6.
Write the structures of: Trichloroethanoic acid, Neopentane, p-nitro benzaldehyde. [Mar. ’13]
Answer:
Trichloroethanoic acid – CCl3 COOH
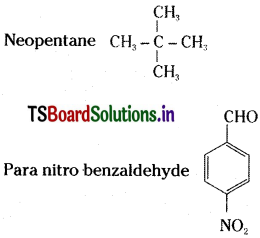
Question 7.
Discuss Lassaigne’s test.
Answer:
Lassaigne’s Test:
In Lassaigne’s test, the organic compound is mixed with sodium metal and fused strongly. The fused mass is extracted with water by plunging the red hot ignition tube in distilled water and the contents are boiled for 5 minutes and filtered. The filtrate is called Sodium extract or Lassaigne’s extract.
If nitrogen is present in the organic compound, it reacts with sodium and carbon forming sodium cyanide.

If sulphur is present in the organic compound, it reacts with sodium forming sodium sulphide.
2 Na + S → Na2S
If halogens are present in the organic compound, they form sodium halides.
2 Na + X → 2NaX (X = Cl, Br or l)
Question 8.
Explain the principle of chromatography.
Answer:
Chromatography is a method of separation of components of a mixture between the stationary phase and a mobile phase.
Chromatography involves the following three steps:
- Adsorption and retention of a mixture of substances on the stationary phase and separation of adsorbed substances by the mobile phase to different distances on the stationary phase,
- Recovery of the substances separated by a continuous flow of the mobile phase (known as elution) and
- Qualitative and quantitative analysis of the eluted substances.
Based on the physical states of the stationary phase and mobile phase and also on the basis of the principle of adsorption of substances on the stationary phase chromatography processes are classified into several types.
Question 9.
Explain why an organic liquid vaporizes at a temperature below its boiling point in its steam distillation.
Answer:
In steam distillation the liquid boils when the sum of vapour pressures due to organic liquid (p1) and that due to water (p2) becomes equal to the atmospheric pressure (p) i.e., p = p1 + p2. Since p1 is lower than p the organic liquid vapourise at lower temperature than its boiling point.
![]()
Question 10.
Explain the following:
a) Crystallisation
b) Distillation.
Answer:
a) Crystallisation :
It is based on the difference in the solubilities of the compound and impurities. Cooling of saturated solution of the compound the solid compound crystallises out of the solution while the impurities being unsaturated remain in solution.
b) Distillation:
Liquids having different boiling points vapourise at different temperatures. The vapours are cooled and the liquids so formed are collected separately.
Short Answer Questions
Question 11.
Complete the following reaction and name the products A, B and C. [AP ’16; TS ’16, 15; IPE ’14]
![]()
Answer:

A is acetylene (or) ethyne. B is benzene. C is toluene or methylbenzene.
Question 12.
Name the products A, B and C formed in the following reactions. Give the equations for the reactions. [TS ’15; Mar. ’09]
![]()
Answer:
A is 1, 2 – dibromoethane.
B is acetylene.
C = 1, 1, 2, 2 – tetra bromoethane.

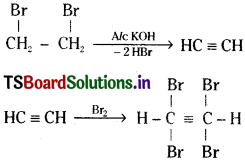
Question 13.
How does acetylene react with : a) Bromine b) Hydrogen? Write the balanced equations for the above reactions. Name the products. asmm
Answer:
a) Acetylene react with bromine in the presence of CCl4 forming an addition compound 1, 1, 2, 2 – tetra bromoethane.
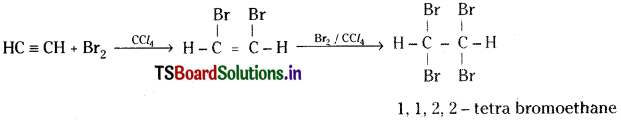
b) Hydrogen react with acetylene in the presence of Ni or Pt catalyst forming ethane.
![]()
Question 14.
What is substitution reaction? Explain any two substitution reactions of benzene. [May ’10]
Answer:
Substitution reaction :
Reactions in which an atom or group present in a compound is replaced by another atom or group are known as ‘substitution reactions’.
Substitution reactions of Benzene :
a) Benzene reacts with bromine or chlorine in presence of AlCl3 to give the corresponding halo benzene.
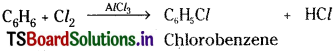
b) Benzene on heating with nitration mixture below 60°C gives nitrobenzene.
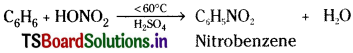
Question 15.
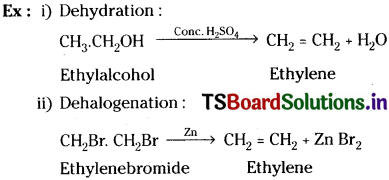
What is dehydrohalogenation? Write the equation for the formation of alkene from alkyl halide.
Answer:
Remoyal of one hydrogen atom and one halogen atom from adjacent carbon atoms of a compound is called dehydrohalogenation.
Alkene from alkyl halide :
Ethyl chloride on heating with ale. KOH undergoes dehydrohalogenation and gives ethylene.
![]()
Question 16.
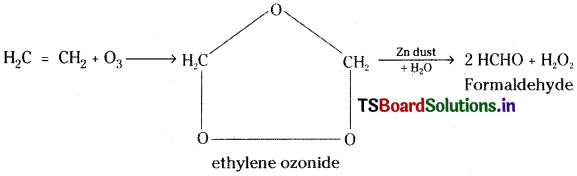
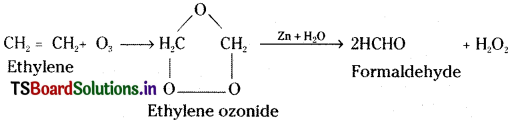
Which type of compounds react with Ozone? Explain with one example. [IPE ’14]
Answer:
Ozonolysis:
Unsaturated hydrocarbons undergo addition with ozone and form addition compounds called ozonides. These ozonides are unstable and undergo hydrolysis forming carbonyl compounds. This is called ozonolysis.
Unsaturated hydrocarbons usually react with ozone. This is used to locate multiple bonds in unsaturated compounds like alkenes, alkynes and benzene.
Eg: Ethylene undergoes addition with ozone forming ethylene ozonide. This on hydrolysis in presence of Zn dust gives formaldehyde and H2O2.
Question 17.
Give two examples each for position and functional isomerism. [TS Mar. 16 ; AP, 16; Mar. 13]
Answer:
Position isomerism is due to difference in the position of a substituent or multiple bond or functional group.
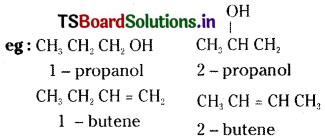
Functional isomerism is due to difference in the functional group in the molecules.
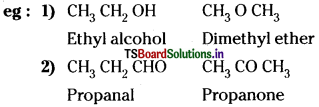
Question 18.
Explain the mechanism of halogenations of methane.
Answer:
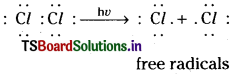
Chlorination of ethane takes place in three steps known as (1) Chain Initiation (2) Chain Propagation and (3) Chain Termination.
1) Chain initiation :
In this step Cl2 molecule absorbs energy and splits into Cl free radicals. (C – C bonds and C – H bonds of ethane being relatively stronger, they do not break at this stage.)
2) Chain propagation:
Chlorine free radicals react with ethane molecule.
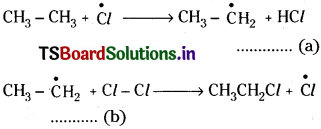
(a) and (b) repeat several times making the reaction a chain reaction and these steps are called propagation steps. In these steps the main products are formed.
In addition to (a) and (b) other reactions to replace other hydrogen atoms of ethane also take place.
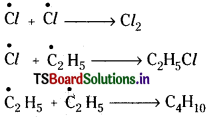
3) Chain termination :
When free radicals directly combine, the chain reaction stops down.
Question 19.
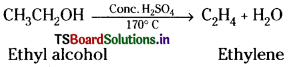
How is ethylene prepared from ethyl alcohol? Write the reaction.
Answer:
Methods of preparation of Ethylene :
1) Dehydration of ethyl alcohol :
Ethyl alcohol on heating with conc. H2SO4 at 170°C given ethylene.
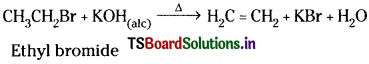
2) Dehydrohalogenation of ethyl halides:
Ethyl halides on heating with alcoholic NaOH or KOH give ethylene.
Question 20.
Explain the reactions of acetylene with :
a) Na in NH3 & b) Chromic acid.
Write the equations and name the products.
Answer:
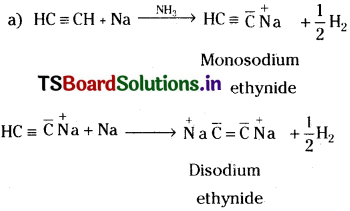
b) Chromic acid oxidises acetylene to acetic acid.
![]()
Question 21.
Explain crystallization and sublimation phenomena which are used in the purification of organic compounds.
Answer:
1) Crystallisation :
The compound which is to be purified is leached with a suitable solvent in a glass container. Then some impurities dissolve and others do not dissolve. Now, the container is heated. The compound starts dissolving and the solubility of the compound increases with increase in temperature. The solution is concentrated to near saturation and filtered while it is hot. On cooling the solution slowly, the compound crystallises out. The crystals are separated by filtration under reduced pressure using Buckner funnel. The soluble impurities remain with the solution. Coloured impurities are removed by adsorbing them with activated charcoal.
Sublimation :
Some solid substances on heating directly pass into vapour state without melting. Those vapours on cooling form directly solid without condensing to liquid. This phenomenon is called sublimation.
![]()
The compound to be purified is taken in a beaker covered with a watch glass and heated. The compound sublimes and solidifies on the lower surface of the watch glass. Impurities remain in the beaker. The compound is separated by scratching the watch glass.
Question 22.
Describe solvent extraction method to purify a compound.
Answer:
Suppose that an organic compound A is more soluble in an organic solvent than in water but present in aqueous solution. The aqueous solution is shaken with the organic solvent. Then A goes into the organic solvent which is immiscible with water. The organic layer is separated and distilled to remove the liquid solvent. The compound A remains in distillation flask.
![]()
Question 23.
Explain the estimation of phosphorous and sulphur in the given organic compounds.
Answer:
Estimation of sulphur :
A known mass of the organic compound is heated with sodium peroxide in a Carius tube. Then ‘S’ is oxidised to H2SO4. The acid is precipitated as BaSO4 by adding excess of BaCl2 aq. solution. The ppt. is filtered, washed, dried and weighed.
Calculations:
Mass of organic compound = a g
Mass of barium sulphate = b g
Molecular weight of BaSO4 = 233
1 mole of BaSO4 or 233 g of BaSO4 contains 32 g of sulphur.
∴ ‘b’ g of BaSO4 contains ……………………. ?
= \(\frac{32\timesb}{233}\) g of sulphur
a g of organic compound contains \(\frac{32\timesb}{233}\) g of sulphur
∴ 100 g organic compounds ” ……………. ?
\(\frac{100\times32\timesb}{a\times233}\) g of sulphur (% of S)
Estimation of phosphorus :
A known mass of organic compound is heated with fuming nitric acid in a Carius tube. Then phosphorus is oxidised to phosphoric acid. The acid is precipitated as ammonium phosphomolybdate by adding ammonia and ammonium molybdate solutions.
Calculation:
Mass of organic compound = a g.
Mass of ammonium phosphomolybdate = b g.
Molecular weight of ammonium phosphomolybdate = 1877.
1877 g of ammonium phosphomolybdate contains 31 g of phosphorus
∴ ‘b’ g of …………………………… ?
\(\frac{31\timesb}{1877}\) g of phosphorus.
’a’ g of organic compound contains
g of phosphorus.
∴ 100 g of ” ” ?
\(\frac{100\times31\timesb}{a\times1877}\) g of sulphur (% of P)
![]()
Question 24.
Explain addition of HBr to Propene with the ionic mechanism.
Answer:
Electrophilic addition of HBr to CH3 – CH = CH2
Molecule :
A pair of electrons from the double bond attacks the electrophilic HBr to produce an achiral trigonal Planarcarbocation intermediate. The halide ion (Br–) then adds to either face of the +vely charged carbon forming the alkyl halide.
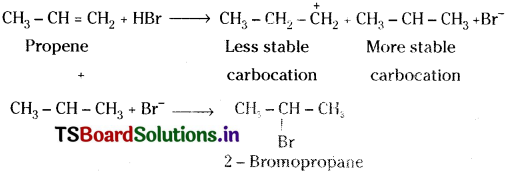
Question 25.
What is the product formed when sodium propionate is heated with soda lime?
Answer:
Decarboxylation of sodium propionate :
Sodium propionate on heating with soda lime (Soda lime = NaOH + CaO) gives ethane.
![]()
Long Answer Questions
Question 26.
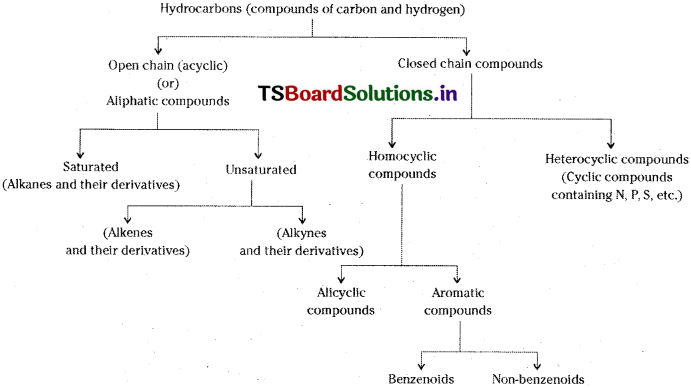
Explain the classification of hydrocarbons.
Answer:
Hydrocarbons are classified as aliphatic hydrocarbons and aromatic hydrocarbons. Aliphatic hydrocarbons are again classified as open chain hydrocarbons and closed chain hydrocarbons i.e., Cyclo hydrocarbons. Both open chain and closed chain hydrocarbons are again classified as hydrocarbons containing
- C – C (alkanes and cycloalkanes)
- > C = C < (alkenes and cycloalkenes)
- – C = C – (alkynes and cycloalkynes)
Question 27.
Write IUPAC names of the following compounds. [AP ’16]
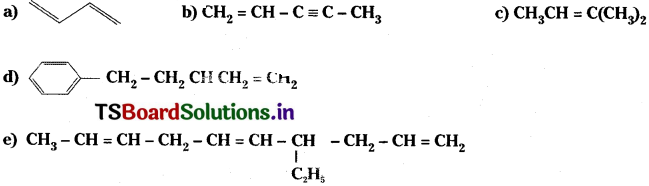
Answer:
a) 1, 3 – Buta diene
b) Pent 1 – ene – 3 yne
c) 2 – methyl, 2 – butene
d) 4 – phenyl, 1 – butene,
e) 4 – methyl deca 1, 5, 8- triene.
Question 28.
Describe two methods of preparation of ethane. Give any three reactions of ethane. [AP Mar. ’19]
Answer:
Methods of preparation of ethane : [Mar. ’13]
1) Decarboxylation of sodium propionate :
Ethane is prepared by heating sodium propionate with soda lime. (Soda lime = NaOH + CaO)
![]()
2) Wurtz reaction:
Ethane is prepared by heating methyl iodide with sodium metal in dry ether. [AP Mar. ’17; TS ’15]
![]()
Properties of ethane:
1) Substitution reactions :
Reactions in which an atom or group present in a compound is replaced by another atom or group are known as substitution reactions,
a) Halogenation:
In the presence of sunlight or UV radiation ethane undergoes halogenation. The H atoms of alkane are successively replaced by the halogen atoms.
![]()
b) Nitration :
Ethane reacts with vapours of HN03 at 400°C and forms nitroethane.
![]()
2) Combustion :
Complete oxidation of the compound in air is called combustion’. It gives CO2 and H2O along with the liberation of heat.
C2H6 \(\frac{7}{2}\)O2 → 2 CO2 + 3 H2O + energy
![]()
Question 29.
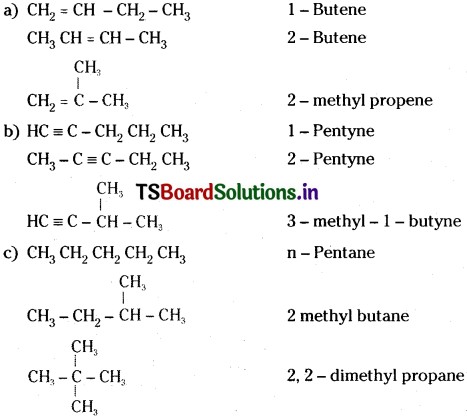
Write the structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated :
a) C4H8 (one double bond)
b) C5H8 (one triple bond)
c) C5H12 (no multiple bonds)
Answer:
Question 30.
Write chemical equations for combustion reaction of the following hydrocarbons,
a) Butane
b) Pentene
c) Hexyne
Answer:
a) C4H10 + \(\frac{13}{2}\)O2 → 4 CO2 + 5H2O
b) C5H10 + \(\frac{15}{2}\)O2 → 5 CO2 + 5 H2O
c) C6H10 + \(\frac{17}{2}\)O2 → 6 CO2 + 5H2O
Question 31.
Addition HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Answer:
HBr directly add to the propene, according to Markovnikov’s rule. While in the presence of benzoyl peroxide HBr adds to propene according to anti Markovnikov rule.
Markovnikov’s rule :
This rule states that when an unsymmetrical reagent adds to a double bond, the +ve part of the adding reagent attaches itself to a carbon of the double bond so as to give the more stable carbocation as an intermediate.
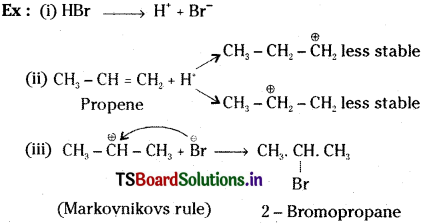
Anti Markovnikov’s rule (or) Kharasch effect:
In presence of peroxide, addition of hydrogen halides to an unsymmetrical alkene takes place in such a way that the hydrogen atom becomes attached to the carbon atom having the fewer number of hydrogen atoms.

Question 32.
Describe two methods of preparation of ethylene. Give equation for the reactions of ethylene with the following. [AP ’16]
a) Ozone [AP Mar. ’19]
b) Hypohalous acid
c) Cold and dil.alk. KMnO4 [AP Mar. ’19; TS Mar. ’18]
d) Heated with O2 at high pressure [AP Mar. ’17]
Answer:
Methods of preparation of Ethylene:
(1) Dehydration of ethyl alcohol:
Ethyl alcohol on heating with cone. H2SO4 at 170°C gives ethylene.

(2) Dehydrohalogenation of ethyl halides:
Ethyl halides on heating with alcoholic NaOH or KOH give ethylene.
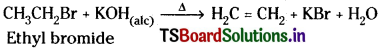
Reactions of Ethylene:
(a) With Ozone :
Ethylene undergoes addition with ozone to form ethylene ozonide. In the presence of (Zn + H2O) this ozonide reduces to carbonyl compounds. The overall process is known as ozonolysis.
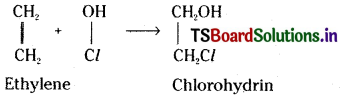
(b) With hypohalous acid :
Ethylene chlorohydrin is formed with hypochlorous acid (HOCl).
(c) With cold dil. alk. KMnO4 :
Ethylene reacts with cold dil. alk. KMnO4 at 273K and forms ethylene glycol.
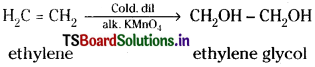
This reaction is used to detect carbon-carbon double bond or triple bond and is known as Baeyer’s test.
(d) Heated with O2 at high pressure : Polyethylene is formed.

Question 33.
How does ethylene react with the following reagents? Give the chemical equations and names of the products formed in the reactions.
a) Hydrogen halide
b) Hydrogen
c) Bromine
d) Water
e) Oxygen in presence of Ag at 200°C
Answer:
a) Ethylene undergoes addition with hydrogen halides forming ethyl halides.
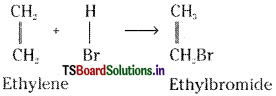
b) Ethylene reacts with hydrogen in presence of Pt, Pd.or Ni catalyst to form ethane.
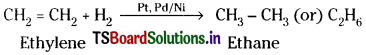
c) Ethylene reacts with bromine in presence of CCl4 to form 1, 2- dibromoethane.
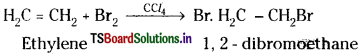
d) When ethylene gas is passed through dil. H2SO4, it undergoes addition with water forming
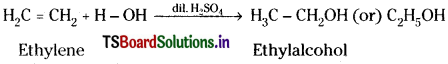
e) Ethylene on heating in oxygen in presence of Ag at 200°C give ethylene oxide.

Question 34.
An alkene ‘A’ on ozonolysis gives a mixture of ethanal and pentan-3-one. Write the reaction, structure of the products and alkene-A. Give the IUPAC name of alkene-A.
Answer:

IUPAC name of A is 3 – ethyl – 2 – pentene.
Question 35.
An alkene ‘A’ contains three C – C, eight C – H bonds and one C = C bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44. Write IUPAC name of ‘A’.
Answer:
The aldehyde with molar mass 44 is CH3 CHO (ethanal). The compound A is
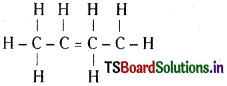
This contains 2 C – C, eight C – H and one C = C bonds. Its IUPAC name is 2 – butene.
![]()
Question 36.
Give two methods of preparation of acetylene. How does it react with water and Ozone? [AP, TS ’15]
Answer:
Methods of preparation of Acetylene :
1) Dehydrohalogenation :
Dibromoethane on heating with alcoholic KOH undergoes dehydro-halogenation and gives acetylene.
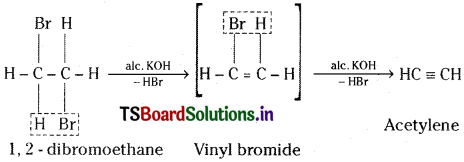
2) From iodoform :
Iodoform on heating with silver powder gives acetylene.
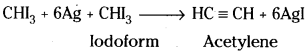
Properties of Acetylene:
1) Action with water:
When acetylene gas is passed through dil. H2SO4 below 60°C in presence of HgSO4, it undergoes addition with water forming acetaldehyde.
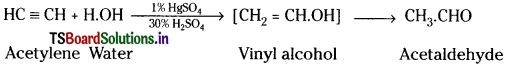
2) Action with ozone :
Acetylene reacts with ozone and undergoes addition to form acetylene ozonide. This on hydrolysis in presence of Zn gives glyoxal.
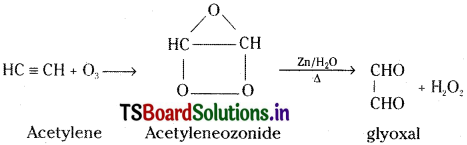
Question 37.
How does acetylene react with the following reagents? Give the corresponding equations and name the products formed in the reactions.
a) Acetic acid
b) Water
c) Hydrogen
d) Halogens
e) Hydrogen halide
f) Ammonical AgNO3 and Cu2Cl2
Answer:
a) Action with CH3COOH :
Acetylene reacts with acetic acid in presence of Hg+2 ions as catalyst, forming at first vinyl acetate and finally ethylidene diacetate.
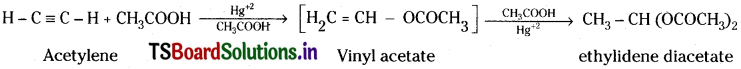
b) Action with water :
When acetylene gas is passed through dil. H2SO4 in presence of HgSO4 below 60°C, it undergoes addition with water forming acetaldehyde.
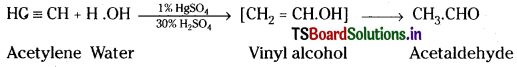
c) Action with hydrogen :
Acetylene reacts with H2 in presence of Ni or Pt catalyst and undergoes addition reaction, forming ethylene and ethane.
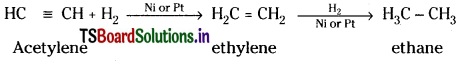
d) Action with halogens :
Acetylene reacts with halogens in presence of CCl4 and undergoes addition reaction giving finally 1, 1, 2, 2 – tetrachloroethane.
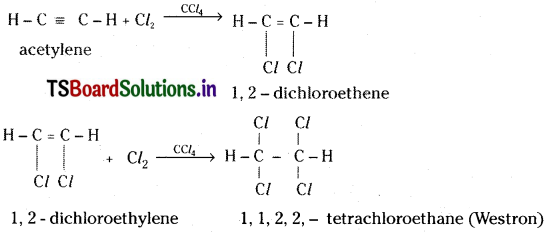
e) Action with hydrogen halides :
Acetylene undergoes addition with hydrogen halides and gives finally 1, 1 — dichloroethane.
f) Action with ammonical AgNO3 solution :
When acetylene gas is passed through ammonical AgNO3 solution, a white ppt. of silver acetylide is formed.

g) Action with ammonical Cu2Cl2 solution :
When acetylene gas is passed through ammonical Cu2Cl2 solution, a red ppt. of cuprous acetylide is formed.

Question 38.
Describe any two methods of preparation of benzene with corresponding equations. Benzene does not behave like an alkene, why? How do we get methyl benzene from benzene? [TS Mar. ’19; Mar. ’18(AP)]
Answer:
Preparation of Benzene :
1) Laboratory Method :
Sodium benzoate on distillation with soda lime gives benzene.
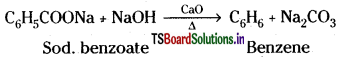
A mixture of NaOH and CaO is called soda lime.
2) Polymerisation of acetylene :
When acetylene gas is passed through red hot copper tube, it undergoes polymerisation forming benzene.

Reason for not behaving as an alkene :
Benzene has a number of resonance structures.
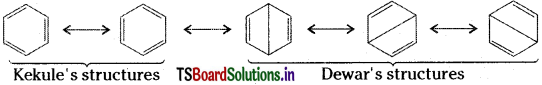
Because of resonance, benzene has more stability. The resonance energy of benzene is also very high. It also indicates more stability to the benzene molecule. Hence, stability of a molecule is due to saturation. Hence, from these two points it is evident that benzene exhibits more the properties of a saturated compound (alkane) rather than the properties of an unsaturated compound (alkene).
Methyl benzene from benzene :
In presence of anhydrous AlCl3, benzene reacts with methyl chloride and forms methyl benzene (Toluene).
Question 39.
How do we get benzene from acetylene? Give the corresponding equation. Explain the halogenation, alkylation, acylation, nitration and sulphonation of benzene. [AP ’15]
Answer:
Preparation of benzene from acetylene :
When acetylene gas is passed through red hot copper tube, it undergoes polymerisation forming benzene.
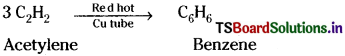
Properties:
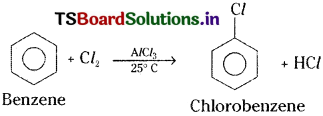
1) Halogenation :
In presence of anhydrous AlCl3, benzene reacts with Cl2 and gives chlorobenzene. [Mar. ’18 (AP); (IPE ’14)]
2) Friedel – Craft’s alkylation :
In presence of AlCl3, benzene reacts with alkyl halides and gives alkyl benzene. This is known as Friedel Craft’s alkylation.
3) Friedel – Craft’s acylation:
In presence of AlCl3, benzene reacts with acetyl chloride and gives acetophenone. [TS Mar. ’19]

4) Nitration :
Benzene reacts with nitration mixture below 60°C and gives nitro-benzene. [AP Mar. ’17]

A mixture of cone. H2SO4 and cone. HNO3 in 1 : 1 ratio (by volume) is called nitration mixture.
5) Sulphonation :
Benzene reacts with fuming H2SO4 and gives benzenesulphonic acid.
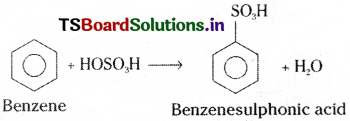
Question 40.
Explain the differences between structural isomers and stereoisomers.
Answer:
Differences:
| Structural Isomers | Stereo Isomers |
| 1. The compounds having same molecular formula but differ in their structural arrangements of atoms of group of atoms. | 1. The compounds having same molecular formula but differ in their spatial arrangement of atoms or group of atoms. |
| 2. Spatial arrangement is not considered. | 2. Structure of the compound is not changed. |
| 3. Structural isomers possess different physical properties. Ex : With increase in number of branches in alkanes with same molecular formula, boiling points are decreased. |
3. Stereo isomers may or may not differ in their physical properties. Ex : a) Diastereomers and cistrans isomers differ in their boiling and melting points. b) Enantiomers have same physical properties. |
| 4. Chain isomers, positional isomers, functional isomers, metamers, ketoenol tautomers and ring isomers are called structural isomers. | 4. Configurational and conformational isomers are called stereoisomers. |
![]()
Question 41.
What is the difference between conformation and configuration in open chain molecules.
Answer:
Stereoisomers are of two types :
- Configurational isomers
- Conformational isomers.
Difference :
Configurational isomers possess certain types of rigidity in the molecules. These molecules can be interconverted only by bond breaking and reforming of new bonds.
Conformations can be easily interconverted by simply rotating about C – C sigma bonds.
Explanation:
Configurational isomers are of two types :
A) Geometrical isomers and
B) Optical isomers.
A) Geometrical isomers :
This is due to the difference in the spatial arrangement of groups about the doubly bonded carbon atoms in the molecules.
Ex : Cis – 2 – Butene and Trans – 2 – Butene
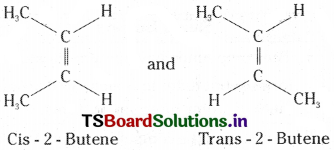
B) Optical isomers:
This is due to the different spatial arrangements of four different atoms or radicals in the molecule.
Ex : ( + ) Lactic acid (1) and ( – ) Lactic acid (2)
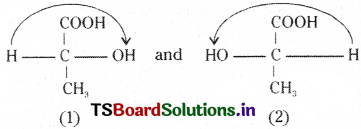
Conformations :
As a result of rotation about single C – C bond, the atoms or groups of a molecule assume different spatial arrangements. These different atomic arrangements are called ‘conformations’.
Ex : Eclipsed (E) and staggered (S) conformations of ethane
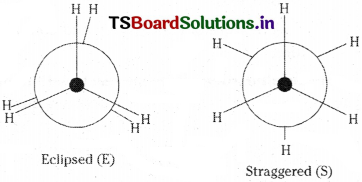
Question 42.
What do you understand about geometrical isomerism? Explain the geometrical isomers of 2 – butene.
Answer:
Geometrical isomerism arises due to a difference in the arrangement of atoms or groups around doubly bonded carbon atoms. 2 – Butene has the following two structures.
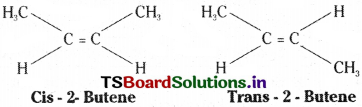
The isomer in which same groups or atoms are on the same side of the C = C is called cis – isomer while the isomer having same groups or atoms on the opposite sides of the C = C is called trans – isomer. In Cis-2- Butene the two methyl groups are on one side while the two hydrogen atoms are on other side. In trans-2-butene two methyl groups and two hydrogen atoms are present opposite to the double bond.
Question 43.
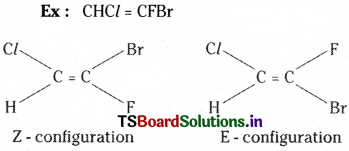
Explain the method of writing E-Z configurations for geometrical isomers taking CHCl – CFBr as your example.
Answer:
The E-Z configuration system for geometrical isomers is based on atomic number ranking method. According to this when atoms of higher atomic number are on the same side of the double bond, the double bond is said to have Z – configuration. When atoms of higher atomic number are on opposite sides of the double bond it is said to have E – configuration.
Question 44.
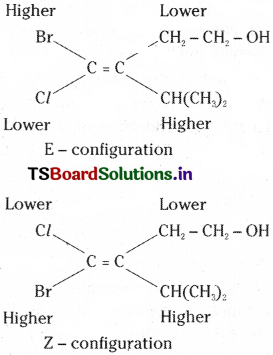
If an alkene contains on carbons at double bond Cl. Br, -CH2 – CH, – OH and -CH(CH3)2. Write the E and Z configurations of it.
Answer:
Question 45.
Write a note on :
a) Distillation
b) Fractional distillation
c) Distillation under reduced pressure
d) Steam distillation
Answer:
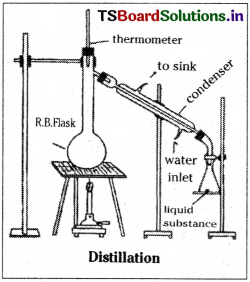
a) Distillation :
This process is useful for the purification of liquids contaminated with non-volatile impurities. The impure liquid is boiled in a distillation flask and the vapours are condensed and collected in a receiver. This method can also be used to separate liquids which differ in their boiling points by about 40°C.
b) Fractional Distillation:
In this process loop tubes having different shapes and designs to fit for a particular requirement are used. These are called fractionating columns. The liquid mixture is taken in a distillation flask, fitted with a fractionating column at its mouth. At the upper end of the column, it is connected to a water condenser. Then vapours of that liquid with lower BP condense and collect in the receiver.
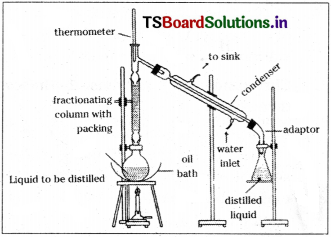
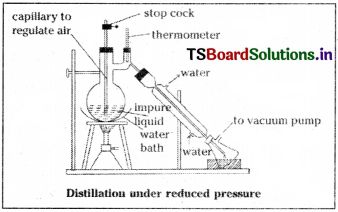
c) Distillation under reduced pressure:
This method is useful to purify liquids that have very high BPs and those which decompose at or below their boiling points. If the external pressure is reduced the liquid boils at lower temperature than its normal BP without decomposition.
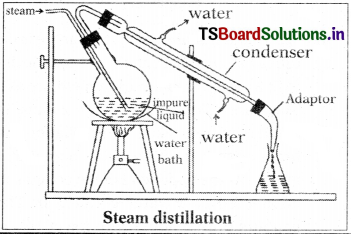
d) Steam distillation:
Liquids immiscible with water and possessing high BP can be purified by steam distillation. In this method, steam is passed into the hot impure liquid. Steam mixed with volatile organic compound comes out. It passes through the condenser and collects in the receiver. The water layer and the organic layer are separated using a separating funnel.
Question 46.
Write a brief note on chromatography.
Answer:
Chromatography is a method of separation of components of a mixture between the stationary phase and a mobile phase.
Chromatography involves the following three steps:
- Adsorption and retention of a mixture of substances on the stationary phase and separation of adsorbed substances by the mobile phase to different distances on the stationary phase.
- Recovery of the substances separated by a continuous flow of the mobile phase (known as elution) and
- Qualitative and quantitative analysis of the eluted substances.
Based on the physical states of the stationary phase and mobile phase and also on the basis of the principle of adsorption of substances on the stationary phase chromatography processes are classified into several types as given below.
| Chromatography processes | Stationary phase | Mobile phase |
| 1) Column chromatography |
solid | liquid |
| 2) Liquid – liquid partition chromatography |
liquid | liquid |
| 3) Paper chromatography | liquid | liquid |
| 4) Thin layer chromatography (TLC) |
liquid or solid | liquid |
| 5) Gas – liquid chromatography (GLC) |
liquid | gas |
| 6) Gas – solid chromatography (GSC) |
solid | gas |
| 7) Ion exchange chromatography |
solid | liquid |
There are two chromatography techniques (1) Adsorption chromatography (2) Partition chromatography.
There are again two types of Adsorption chromatography techniques. They are (1) Column chromatography (2) Thin layer chromatography.
![]()
Question 47.
Explain the following.
a) Column chromatography
b) Thin layer chromatography
c) Partition chromatography
Answer:
a) Column chromatography :
In this type of chromatography the components of a mixture are separated by a column of adsorbent (stationary phase) packed in a glass tube. The column is fitted with a stopcock at its lower end. The mixture to be adsorbed on the adsorbent is placed at the top of the stationary phase. A suitable eluant (solvent) is allowed to flow down the column slowly. Depending on the degree to which the compounds are adsorbed, the components are separated. The most readily adsorbed substances are retained near the top and others come down accordingly to various distances.
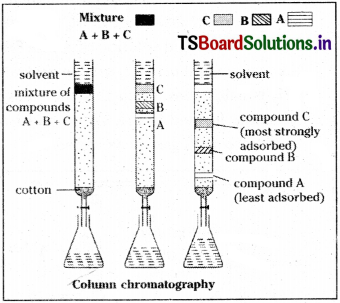
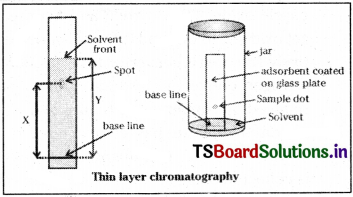
b) Thin layer chromatography :
This type of chromatography also involves adsorption differences. Here the adsorbent silica gel or alumina is coated over a glass plate of suitable size. The plate is called TLC plate or chromoplate. The solution of the mixture to be separated is applied as a small spot at about 2 cm from the bottom of the plate. The plate is then kept in a closed jar containing the eluant (solvent). As the eluant rises up the plate, the components of the mixture move up along with the eluant to various distances depending on their degree of adsorption.
The relative adsorption of a component of the mixture is expressed in terms of its Retardation Factor (Rf) value.

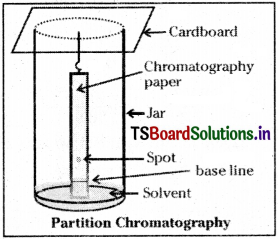
c) Partition chromatography :
This is based on continuous differential partitioning of components of a mixture between the stationary phase and the mobile phase. In this type of chromatography, a special paper called chromatography paper contains water trapped in it which acts as the stationary phase. The chromatography paper spotted with the solution of the mixture at the base is suspended in a suitable solvent or a mixture of solvents. This solvent acts as the mobile phase The solvent rises up the paper by capillary action and moves over the spot.
The paper selectively retains different components as per their differing partition in mobile and stationary phases. The paper strip so developed is known as chromatogram. The spots of the separated coloured compounds are detected. For colourless compounds other methods like spraying a suitable reagent are used.
Question 48.
Explain the estimation of nitrogen of an organic compound by
a) Dumas method
b) Kjeldahl’s method
Answer:
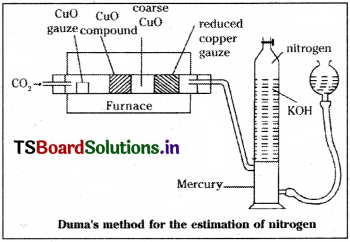
a) Estimation of nitrogen by Duma’s method :
In this method, a known weight of organic compound is heated strongly with coarse cupric oxide. Then carbon and hydrogen get oxidised to CO2 and H2O vapour respectively. Nitrogen is converted to N2 gas. Some nitrogen converted into its oxides, gets reduced by copper gauze to nitrogen. The liberated gases are passed over a solution of KOH. Then CO2 gets absorbed. Nitrogen is collected over KOH solution and its volume is found out.
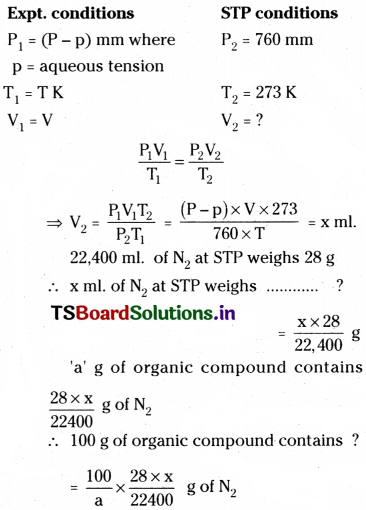
Calculation :
Suppose ‘a’ g of organic compound gives V ml of N2 gas at room temp. T K.
Vol. of N2 gas at STP is calculated as follows.
b) Estimation of N2 by Kjeldahl’s method :
In this method the organic compound is heated with cone. H2SO4 in presence of a small amount of CuSO4. Then N2 is quantitatively converted into ammonium sulphate. The contents of the flask are transferred into another flask and heated with excess of NaOH solution to liberate ammonia gas. The gas so liberated is passed and absorbed in a known vol. of known cone. H2SO4 (excess). Now the excess of acid remained after the neutralisation by NH3 is titrated against a standard solution of alkali. From this, the amount of H2SO4 used to neutralise NH3 is calculated. From this the mass of ammonia formed is calculated and from that, the % of N2 gas is calculated.
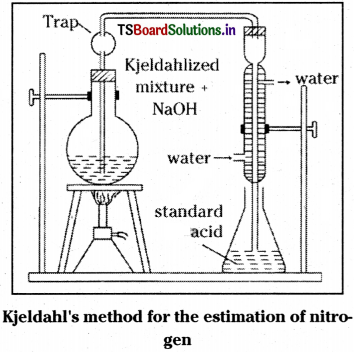
Organic compound + H2SO4 → (NH4)2 SO4
(NH4)2SO4 + 2 NaOH → Na2SO4 + 2 H2O + 2NH3
2 NH3 + H2SO4 → (NH4)2SO4
Calculations:
Mass of organic compound = a g
Vol. of H2SO4 initially taken = V ml.
Molarity of H2SO4 = M
Vol. of NaOH consumed after complete neutralisation = V1 ml.
Molarity of NaOH = M
2NaOH + H2SO4 → Na2SO4 + 2 H2O
Then from the formula,
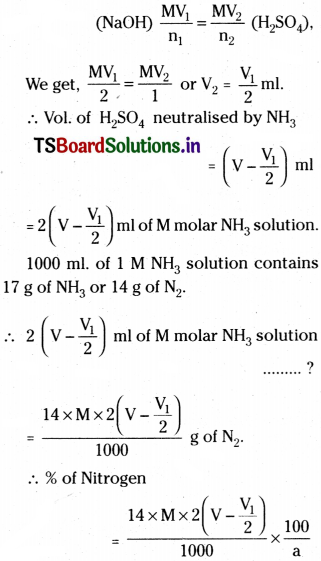
Question 49.
Explain inductive effect with a suitable example.
Answer:
Consider, the molecule CH3 – CH2 – CH2 – Cl. There is a o bond between ‘C’ atom and ‘Cl’ atom. The electron pair between them is not equally shared. The more electro -ve ‘Cl’ atom tends to attract the shared pair more towards itself. Due to this, the electron density tends to be greater near the ‘Cl’ atom than ’C’ atom. It is represented as – C ![]() Cl. But carbon atom bonded to chlorine atom is itself attached to other carbon atoms. Therefore the effect can be transmitted further.
Cl. But carbon atom bonded to chlorine atom is itself attached to other carbon atoms. Therefore the effect can be transmitted further.
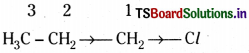
The effect of the ‘Cl’ atom is to leave C1 slightly electron deficient. C1 in turn tries to rectify this by appropriating slightly more than its share of electrons of the σ bond joining it to C2 and this continues down the carbon chain. However, the effect of C1 on C2 is less than the effect of Cl on C1 and the transmission quickly falls down away in a saturated chain and is generally too small to be noticeable beyond C3. These influences on the electron distribution in o bonds are known as inductive effects.
Thus, inductive effect can be defined as “The electron-donating or electron-withdrawing effect of an atom or group of atoms that is transmitted by the polarisation of electrons in a bonds”.
Inductive effect is a measure of the ability of substituents to either withdraw or donate electron density to the attached carbon atom. Based on this ability, the substituents are classified as electron-withdrawing or electron-donating groups relative to hydrogen. Electron-withdrawing groups are said to exhibit – ve inductive effect (-1) and electron-donating groups are said to exhibit + ve inductive effect (+1).
Inductive effect influences chemical reactivity and physical properties.
![]()
Question 50.
Write a note on mesomeric effect.
Answer:
“The electron pair displacement caused by an atom or group of atoms along a chain by a conjugative mechanism is called the Mesomeric effect of that atom or group”. Salient features of mesomeric effect (M):
(a) This is a permanent effect operating in the ground state of the molecule.
(b) Lone pairs and π electrons are involved and operate through conjugative mechanism of electron displacement.
(c) It influences the physical properties, reaction rates etc.
Groups which tend to increase the electron density of the rest of the molecule are said to have +M effect. Such groups tend to possess lone pairs of electrons.
![]()
Groups that decrease the electron density of the rest of the molecule are said to have -M effect. Unsaturated groups having polar character have – M effect.

Here C = O group decreases the electron density of the remaining molecule. It has -M effect.
Question 51.
Describe Resonance effect with one example.
Answer:
It is not possible to explain the properties of a molecule by giving a single structure to it. Then several possible structures are to be proposed. Each structure will explain some properties of the molecule. All structures put together explain all the properties. This phenomenon is known as resonance. All the structures thus proposed are called resonance or canonical structures.
Ex : Urea CO(NH2)2 has the following resonance (or canonical) structures.
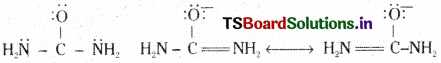
Salient features of resonance :
- Resonance structures are interconvertable.
- Resonance involves only displacement of electrons without disturbing the positions of atoms.
- All atoms in the molecule should lie in the same plane.
- The no. of paired and unpaired electrons should be the same in all the structures.
- The canonical structures should have nearly equal energy.
- More stable resonance structure contributes more to the actual structure of the molecule.
- More the delocalisation of electrons, more is the stability.
- More the covalent bonds, more is the stability and charge separation gives less stability.
Resonance effect:
It is the polarity produced in a molecule by the interactions of two π bonds or between a π bond and a lone pair of electrons present on adjacent atoms. This effect is transmitted throughout the chain.
If the transfer of electrons is away from the atoms of substituent groups attached to the conjugated system, then the molecule gets high electron density in some of its positions as in aniline and it is given (+R). If the shift of electrons is towards the atom or substituent groups, it is (-R) as in nitrobenzene.
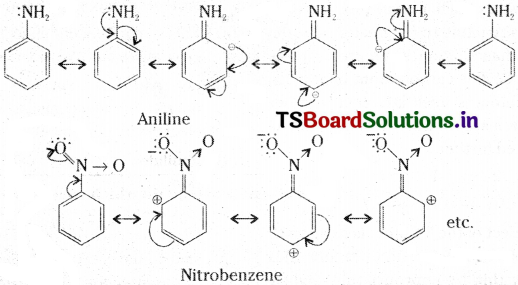
Resonance Energy :
The energy difference between the real structure and the most stable resonance structure is called resonance stabilisation energy or resonance energy.
Question 52.
Explain how many types of organic reactions are possible.
Answer:
Common types of organic reactions are (1) Substitution reactions (2) Addition reactions (3) Elimination reactions (4) Rearrangement reactions.
1) Substitution (Displacement) reactions:
The replacement of an atom or group from a molecule by a different atom or group is known as ‘substitution reaction’.
2) Addition reactions:
Reactions in which atoms or groups of atoms are added to a molecule are known as addition reactions.
The multiple bond breaks and then the addition takes place.
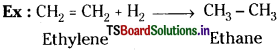
3) Elimination reactions :
The molecule loses atoms or group of atoms and a multiple bond is formed, (these are actually the reverse of addition reactions)
4) Molecular rearrangement reactions :
In these reactions, an atom or a group migrates from one atom to another atom, within the same molecule.
Ex : i) n – Butane to Isobutane :

ii) Benzophenone oxime to Benzanilide :
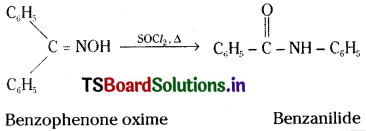
Question 53.
Write the possible conformations of ethane and explain which is more stable. [Mar. ’19]
Answer:
Conformations :
As a result of rotation about single C – C bond, the atoms or groups of a molecule assume different spatial arrangements. These different atomic arrangements are called ‘conformations’.
Ex : Eclipsed (E) and staggered (S) conformations of ethane.
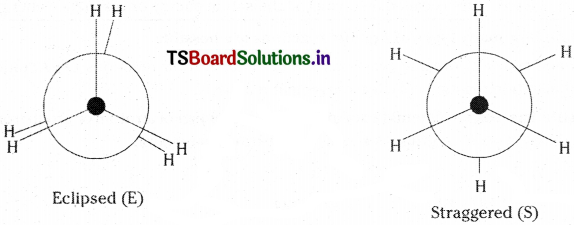
Staggered conformation is more stable than eclipsed because in eclipsed conformation the hydrogen atoms are nearer and repel each other. But in staggered conformation, the hydrogen atoms are at maximum possible distance. So it is more stable.
![]()
Question 54.
Explain aromatic electrophilic substitution reactions of benzene.
Answer:
Electrophilic substitution reaction of benzene proceeds in two steps.
Step 1:
The electrophile (E+) takes two electrons of the six – electron π system, to form a σ bond with a carbon atom of the benzene ring. Then that carbon atom becomes sp³ hybridized.
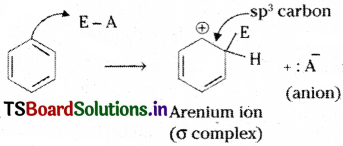
Step 2 :
A proton is removed from the carbon atom of the σ complex that bears the E (ele-ctrophile). Then that carbon atom changes to sp² state again. Then the benzene derivative with fully delocalised six π electrons is formed.
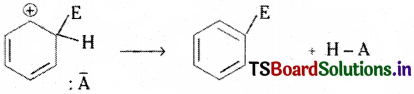
(The proton H+ combines with the anion obtained from the molecule E – A → E+ + : A–)
Question 55.
Explain electrophilic addition reactions of ethylene with mechanism.
Answer:
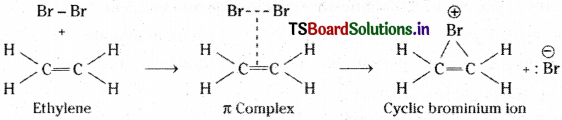
1) Addition of halogens :
Ethylene combines with halogens at room temp, in an inert solvent medium, to give a vicinal dihalide.

Mechanism : It involves 2 steps.
Step (I) :
The π – electrons of the double bond attack the halogen molecule. Then a loose π complex (cyclic bromonium ion), with a 3 – membered ring is formed.
Step (II) :
The negative bromide ion attacks the cyclic bromonium ion from the backside.
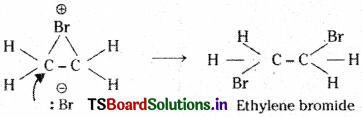
This is called, ’trans’ addition of halogens.
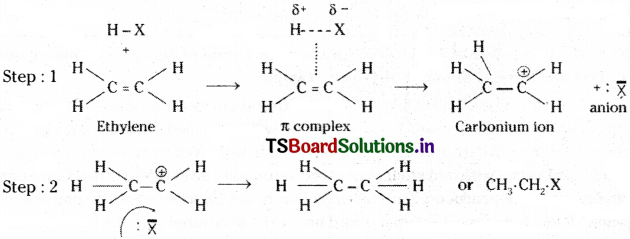
2) Addition of hydrogen halide :
Ethylene reacts with hydrogen halide (HX) to give ethyl halide.

Mechanism: This mechanism is quite similar to that of the halogen addition, involving 2 steps. H-X
3) Addition of hypohalous acid to ethylene :
Ethylene reacts with hypochiorous acid to give ethylene chlorohydrin.
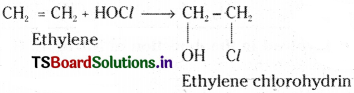
Mechanism : HOCl → (HO)– + Cl+
The same mechanism as in the case of addition of HX.
HX → H+ + X–
In HOCl, the Cl+ adds first. Then OH– adds to the other carbon atom.
Question 56.
With the help of mechanism explain free radical halogenations of alkanes.
Answer:
Chlorination of ethane takes place in three steps known as (1) Chain Initiation (2) Chain Propagation and (3) Chain Termination.
1) Chain initiation :
In this step Cl2 molecule absorbs energy and splits into Cl free radicals. (C – C bonds and C – H bonds of ethane being relatively stronger, they do not break at this stage.)

2) Chain propagation:
Chlorine free radicals react with ethane molecule.

(a) and (b) repeat several times making the reaction a chain reaction and these steps are called propagation steps. In these steps the main products are formed.
In addition to (a) and (b) other reactions to replace other hydrogen atoms of ethane also take place.
3) Chain termination :
When free radicals directly combine, the chain reaction stops down.

Question 57.
Discuss Markovnikov’s rule and Kharash effect.
Answer:
HBr directly add to the propene, according to Markovnikov’s rule. While in the presence of benzoyl peroxide HBr adds to propene according to anti Markovnikov rule.
Markovnikov’s rule :
This rule states that when an unsymmetrical reagent adds to a double bond, the +ve part of the adding reagent attaches itself to a carbon of the double bond so as to give the more stable carbocation as an intermediate.

Anti Markovnikov’s rule (or) Kharasch effect:
In presence of peroxide, addition of hydrogen halides to an unsymmetrical alkene takes place in such a way that the hydrogen atom becomes attached to the carbon atom having the fewer number of hydrogen atoms.

Question 58.
How would you convert the following compounds into benzene?
a) Chlorobenzene
b) Toluene
c) p – nitro toluene
Answer:
a) Reduction of chlorobenzene with Ni – Al alloy and NaOH gives benzene
C6H5 Cl + 2 [H] → C6H6 + HCl
b) Toluene is first oxidised to benzoic acid with dil, HNO3, and alkaline or acidic KMnO4. Then decarboxylation of benzoic acid gives benzene.
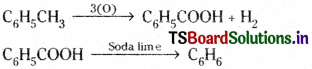
c) Nitro toluene is first reduced to toludine.
![]()
This on diazotization forms diazonium salt which on treating with KCN/Cu followed by hydrolysis gives p-methyl benzoic acid, The methyl group is also oxidised to acid group with dil. HNO3. This on decarboxylation gives benzene.
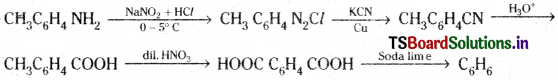
![]()
Question 59.
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate with one example.
Answer:
In Wurtz reaction when alkyl halide is treated with sodium metal, an alkane having double the number of carbon atoms present in alkyl halide will be formed. So always we get an alkane with even number of carbon atoms. If an alkyl halide with even number of carbon atoms and another alkyl halide with odd number of carbon atoms are used in Wurtz reaction a mixture of hydro carbons are produced. So Wurtz reaction is not preferable for the preparation of alkanes with odd number of carbon atoms. This can be illustrated with the following examples.
CH3 Cl + 2 Na + Cl CH3 → CH3 – CH3 + 2NaC7
CH3 CH2 Cl + 2Na + Cl CH2 CH3 → CH3 CH2 CH, CH;i + 2 NaCl
CH3Cl + 2Na + Cl CH2 CH3 → CH3 – CH3 + CH3 CH2 CH3 + CH3 CH2 CH2 CH3
Question 60.
Write the equations involved in the detection of Nitrogen, Halogens and Sulphur in organic compounds.
Answer:
Lassaigne’s Test:
In Lassaigne’s test, the organic compound is mixed with sodium metal and fused strongly. The fused mass is extracted with water by plunging the red hot ignition tube in distilled w’ater and the contents are boiled for 5 minutes and filtered. The filtrate is called Sodium extract or Lassaigne’s extract.
If nitrogen is present in the organic compound, it reacts with sodium and carbon forming sodium cyanide.
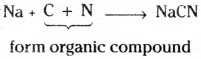
If sulphur is present in the organic compound, it reacts with sodium forming sodium sulphide.
2 Na + S → Na2S
If halogens are present in the organic compound, they form sodium halides.
2 Na + X → 2 NaX (X = Cl, Br or I)
Test for Nitrogen :
A little of the Lassaigne’s extract is made alkaline with NaOH solution and then freshly prepared FeSO4 solution is added. To it 2 – 3 drops of FeCl3 solution is added, cooled and acidified with cone. HCl.
A prussian blue or green ppt. or colouration is observed.

Test for Halogens:
A little of the Lassaigne’s extract is acidified with nitric acid and treated with AgNO3 solution.
If white ppt. soluble in NH4OH is formed, the halide is Cl–. Hence, chlorine is present.
If pale yellow ppt. partially soluble in NH4OH is formed, the halide is Br–. Hence, bromine is present.
If yellow ppt. insoluble in NH4OH is formed, the halide is l–. Hence, iodine is present.
Test for Sulphur :
To a little of the Lassaigne’s extract freshly prepared sodium nitroprus-side solution is added. Deep purple colouration is observed.
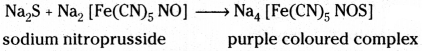
Question 61.
Explain how Carbon and Hydrogen are quantitatively determined in an organic compound.
Answer:
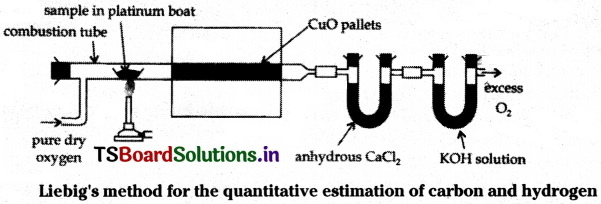
A known weight of the organic compound is taken and completely burnt in excess of air and copper (II) oxide. Then carbon oxidises to CO2 and hydrogen oxidises to H2O. The CO2 and H2O so obtained are passed through already weighed U tubes containing anhydrous CaCl2 and caustic potash respectively. The increased weights of these two tubes give the weights of H2O and CO2 formed.
Suppose that a’ g of organic compound on combustion gives ‘b’ g of water vapour and e g of CO2.
% of carbon:
44 g of CO2 contains 12 g of carbon.
∴ ‘c’ g of CO2 contains ………….. ?
= \(\frac{12\timesc}{44}\) g of carbon
‘a’ g of organic compound contains \(\frac{12\timesc}{44}\) g of carbon.
100 g of organic compound contains ………….. ?
= \(\frac{100\times12\timesc}{a\times44}\) g of carbon (% of C)
% of hydrogen:
18 g of water contains 2 g of H2.
∴ ‘b’ g of water contains ………….. ?
= \(\frac{b\times2}{18}\) g of hydrogen
a g of organic compound contains \(\frac{b\times2}{18}\) g of hydrogen.
∴ 100 g of organic compound contains ………….. ?
= \(\frac{b\times2\timesc}{18\timesa}\) g of hydrogen (% of H)
![]()
Question 62.
Describe Dumas and Kjeldhahl’s method for the estimation of Nitrogen.
Answer:
a) Estimation of nitrogen by Duma’s method :
In this method, a known weight of organic compound is heated strongly with coarse cupric oxide. Then carbon and hydrogen get oxidised to CO2 and H2O vapour respectively. Nitrogen is converted to N2 gas. Some nitrogen converted into its oxides, gets reduced by copper gauze to nitrogen. The liberated gases are passed over a solution of KOH. Then CO2 gets absorbed. Nitrogen is collected over KOH solution and its volume is found out.

Calculation :
Suppose ‘a’ g of organic compound gives V ml of N2 gas at room temp. T K.
Vol. of N2 gas at STP is calculated as follows.

b) Estimation of N2 by Kjeldahl’s method :
In this method the organic compound is heated with cone. H2SO4 in presence of a small amount of CuSO4. Then N2 is quantitatively converted into ammonium sulphate. The contents of the flask are transferred into another flask and heated with excess of NaOH solution to liberate ammonia gas. The gas so liberated is passed and absorbed in a known vol. of known cone. H2SO4 (excess). Now the excess of acid remained after the neutralisation by NH3 is titrated against a standard solution of alkali. From this, the amount of H2SO4 used to neutralise NH3 is calculated. From this the mass of ammonia formed is calculated and from that, the % of N2 gas is calculated.

Organic compound + H2SO4 → (NH4)2 SO4
(NH4)2SO4 + 2 NaOH → Na2SO4 + 2 H2O + 2NH3
2 NH3 + H2SO4 → (NH4)2SO4
Calculations:
Mass of organic compound = a g
Vol. of H2SO4 initially taken = V ml.
Molarity of H2SO4 = M
Vol. of NaOH consumed after complete neutralisation = V1 ml.
Molarity of NaOH = M
2NaOH + H2SO4 → Na2SO4 + 2 H2O
Then from the formula,

Question 63.
How do you determine Sulphur, Phosphorous and Oxygen quantitatively in an organic compound?
Answer:
Estimation of sulphur :
A known mass of the organic compound is heated with sodium peroxide in a Carius tube. Then ‘S’ is oxidised to H2SO4. The acid is precipitated as BaSO4 by adding excess of BaCl2 aq. solution. The ppt. is filtered, washed, dried and weighed.
Calculations:
Mass of organic compound = a g
Mass of barium sulphate = b g
Molecular weight of BaSO4 = 233
1 mole of BaSO4 or 233 g of BaSO4 contains 32 g of sulphur.
∴ ‘b’ g of BaSO4 contains ……………………. ?
= \(\frac{32\timesb}{233}\) g of sulphur
a g of organic compound contains \(\frac{32\timesb}{233}\) g of sulphur
∴ 100 g organic compounds ” ……………. ?
\(\frac{100\times32\timesb}{a\times233}\) g of sulphur (% of S)
Estimation of phosphorus :
A known mass of organic compound is heated with fuming nitric acid in a Carius tube. Then phosphorus is oxidised to phosphoric acid. The acid is precipitated as ammonium phosphomolybdate by adding ammonia and ammonium molybdate solutions.
Calculation:
Mass of organic compound = a g.
Mass of ammonium phosphomolybdate = b g.
Molecular weight of ammonium phosphomolybdate = 1877.
1877 g of ammonium phosphomolybdate contains 31 g of phosphorus
∴ ‘b’ g of …………………………. ?
\(\frac{31\timesb}{1877}\) g of phosphorus.
’a’ g of organic compound contains
g of phosphorus.
∴ 100 g of ” ” ?
\(\frac{100\times31\timesb}{a\times1877}\) g of sulphur (% of P)
Estimation of Oxygen :
A known weight of organic compound is decomposed by heating in a stream of nitrogen gas. The mixture of gaseous products containing oxygen is passed over red hot coke to convert all oxygen in those oxides to CO. Then the mixture is passed through hot iodine pentoxide to convert CO into CO2 and iodine liberates.
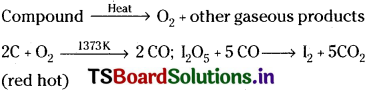
Calculation:
Mass of organic compound = a g
Mass of carban dioxide b g
44 g of CO2 contains 32 g of oxygen.
∴ b g CO2 contains ……………. ?
= \(\frac{b\times32}{44}\) g of oxygen
‘a’ g of organic compound contains \(\frac{b\times32}{44}\) g of O2.
∴ 100 g of organic compound contains ………… ?
= \(\frac{100\timesb\times32}{a\times44}\) g. of oxygen (% of oxygen)
![]()
Question 64.
Explain Carius method for the determination of Halogens quantitatively in an organic compound.
Answer:
Halogens can be estimated by Carius method.
In this method a known weight of the organic compound is heated with fuming nitric acid in presence of AgNO3 in a hard glass tube. Carbon and hydrogen are oxidised to CO2 & H2O. Halogens are converted into silver halides. The silver halide is filtered off, washed, dried and weighed.
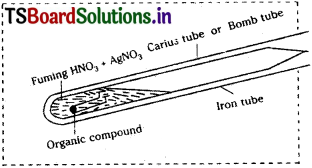
Calculation :
Mass of the organic com-pound = ‘a’ g.
Mass of silver halide formed (AgX) = ‘b’ g.
1 mole of AgX contains 1 mole of X
∴ Mass of halogen in ‘b’ g of AgX ……………… ?
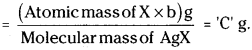
‘a’ g of organic compound has ‘C’ g of halogen.
∴ 100 g of organic compound has …………… ?
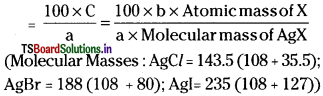
Question 65.
What is carcinogenicity? Explain with two examples.
Answer:
Benzene and several polynuclear hydrocarbons like 1,3- Benzanthracene, 3 – Methyl- cholanthrene, 1,2- Benzpyrene are toxic and said to be carcinogenic (cancer-producing). Most of these are formed due to incomplete combustion of organic substances like tobacco, coal, petroleum etc. They undergo various chemical changes in human body and finally damage DNA to cause cancer.
Additional Questions & Answers
Question 1.
How many σ and π bonds are present in each of the following molecules?
(a) HC ≡ CCH=CHCH3 (b) CH2=C=CHCH3
Answer:
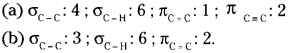
Question 2.
What is the type of hybridisation of each carbon in the following compounds?
(a) CH3Cl, (b) (CH3)2CO, (C) CH3CN, (d) HCONH2, (e) CH3CH=CHCN
Answer:
(a) sp³,
(b) sp³, sp²,
(c) sp³, sp,
(d) sp²,
(e) sp³, sp², sp², sp
![]()
Question 3.
Write the state of hybridisation of carbon in the following compounds and shapes of each of the molecules.
(a) H2C = O, (b) CH3F, (C) HC ≡ N.
Answer:
(a) sp² hybridised carbon, trigonal planar;
(b) sp³ hybridised carbon, tetrahedral;
(c) sp hybridised carbon, linear.
Question 4.
Expand each of the following condensed formulas into their complete structural formulas.
(a) CH3CH2COCH2CH3
(b) CH3CH = CH(CH2)3CH3
Answer:
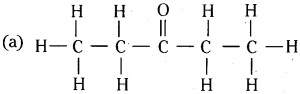
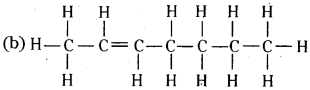
Question 5.
For each of the following compounds, write a condensed formula and also their bond-line formula.
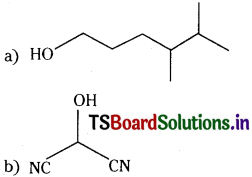
(a) HOCH2CH2CH2CH(CH)3CH(CH3)CH3
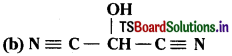
Answer:
Condensed formula:
a) HO(CH2)3CH(CH3)CH(CH3)2
b) HOCH(CN)2
Bond-line formula:
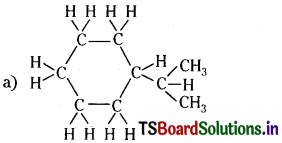
Question 6.
Expand each of the following bond-line formulas to show all the atoms including carbon and hydrogen
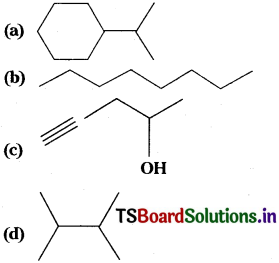
Answer:
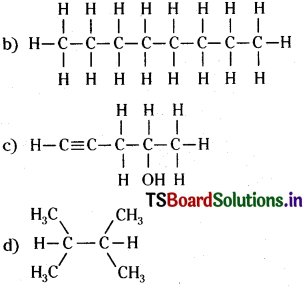
Question 7.
Structures and IUPAC names of some hydrocarbons are given below. Explain why the names given in the parentheses are incorrect.
a)
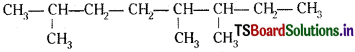
2, 5, 6 – Trimethyloctane [and not 3,4,7 Trimethyloctane]
b)
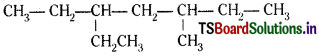
3-Ethyl-5-methylheptane [and not 5-Ethyl- 3-methylheptane]
Answer:
a) Lowest locant number, 2,5,6 is lower than 3,5,7,
b) substituents are in equivalent position; lower number is given to the one that comes first in the name according to alphabetical order.
![]()
Question 8.
Write the IUPAC names of the compounds i-iv from their given structures.
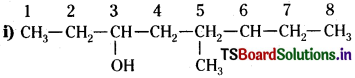
Answer:
The functional group present is an alcohol (OH). Hence the suffix is ‘-ol’
The longest chain containing – OH has eight carbon atoms. Hence the corresponding saturated hydrocarban is octane.
The -OH is on carbon atom 3. In addition, a methyl group is attached at 6th carbon. Hence, the systematic name of this compound is 6-Methyloctan-3-ol.
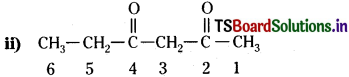
Answer:
The functional group present is ketone (>C=0), hence suffix ‘-one’. Presence of two keto groups is indicated by ‘di’, hence suffix becomes ‘dione’. The two keto groups are at carbons 2 and 4. The longest chain contains 6 carbon atoms, hence, parent hydrocarbon is hexane. Thus, the systematic name is Hexane- 2,4-dione.

Answer:
Here, two functional groups namely ketone and carboxylic acid are present. The principal functional group is the carboxylic acid group; hence the parent chain will be suffixed with ‘oic’ acid. Numbering of the chain starts from carbon of – COOH functional group. The keto group in the chain at carbon 5 is indicated by ‘oxo’. The longest chain including the principal functional group has 6 carbon atoms; hence the parent hydrocarbon is hexane. The compound is, therefore, named as 5-Oxohexanoic acid.
![]()
Answer:
The two C = C functional groups are present at carbon atoms 1 and 3, while the Ca”C functional group is present at carbon 5. These groups are indicated by suffixes ‘diene’ and ‘yne’ respectively. The longest chain containing the functional groups has 6 carbon atoms; hence the parent hydrocarbon is hexane. The name of compound, therefore, is Hexa-1,3- dien-5-yne.
![]()
Question 9.
Derive the structure of (i) 2-Chlorohexane, (ii) Pent-4-en-2-ol, (iii) 3- Nitrocyclohexene, (iv) Cyclohex-2-en-l-ol, (v) 6-Hydroxyheptanal.
Answer:
i) ‘hexane’ indicates the presence of 6 carbon atoms in the chain. The functional group chloro is present at carbon 2. Hence, the structure of the compound is CH3CH2CH2CH3CH(Cl)CH3.
ii) ‘pent’ indicates that parent hydrocarbon contains 5 carbon atoms in the chain, ‘en’ and ‘of’ correspond to the functional groups C=C and -OH at carbon atoms 4 and 2 respectively. Thus, the structure is CH2=CHCH2CH (OH)CH3.
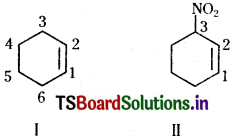
iii) Six membered ring containing a carbon-carbon double bond is implied by cyclo-hexene, which is numbered as shown in (I). The prefix 3-nitro means that a nitro group is present on C-3. Thus, complete structural formula of the compound is (II). Double bond is suffixed functional group whereas NO2 is prefixed functional group therefore double bond gets preference over – NO, group:
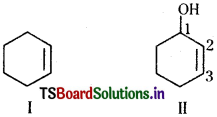
iv) ’l-ol’ means that a -OH group is present at C-l. OH is suffixed functional group and gets preference over C=C bond. Thus the structure is as shown in (II):
v) ‘heptanal’ indicates the compound to be an aldehyde containing 7 carbon atoms in the parent chain-. The ’6-hydroxy’ indicates that -OH group is present at carbon 6. Thus, the structural formula of the compound is :
CH3CH(OH)CH2CH2CH2CH2CHO. Carbon atom of -CHO group is included while numbering the carbon chain.
Question 10.
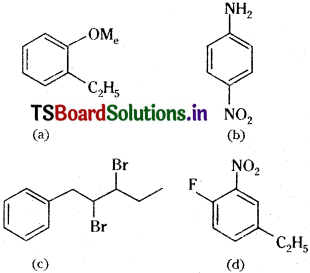
Write the structural formula of:
(a) o-Ethylanisole, (b) p-Nitroaniline,
(c) 2,3 – Dibromo -1 – phenylpentane,
(d) 4-Ethyl-l-fluoro-2-nitrobenzene.
Answer:
Question 11.
Using curved-arrow notation, show the formation of reactive intermediates when the following covalent bonds undergo heterolytic cleavage.
(a) CH3-SCH3, (b) CH3-CN,(c) CH3-CU
Answer:
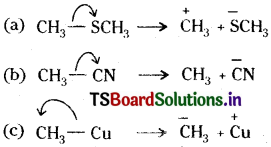
Question 12.
Giving justification, categorise the following molecules/ions as nucleophile or electrophile:
HS–, BF3, C2H5O–, (CH3)3N:,
Cl+, CH3 – C+ =O, H2N:–, N+O2
Answer:
Nucleophiles :
HS–, C2H5O–, (CH3)3N:, H2N–.
These species have unshared pair of electrons, which can be donated and shared with an electrophile.
Electrophiles:
BF3,Cl+,CH3-C = 0, N+O–2.
Reactive sites have only six valence electrons; can accept electron pair from a nucleophile.
Question 13.
Identify electrophilic centre in the following: CH3CH = O, CH3CN, CH3I.
Answeer:
![]()
starred carbon atoms are electrophilic centers as they will have partial positive charge due to polarity of the bond.
![]()
Question 14.
Which bond is more polar in the following pairs of molecules:
(a) H3C-H, H3C-Br
(b) H3C-NH2, H3C-OH
(c) H3C-OH, HSC-SH
Answer:
(a) C-Br, since Br is more polar electro-negative then H,
(b) C-O,
(c) C-O
Question 15.
In which C-C bond of CH3CH2CH2Br, the inductive effect is expected to be the least?
Answer:
Magnitude of inductive effect diminishes as the number of intervening bonds increases. Hence, the effect is least in the bond between carbon-3 and hydrogen.
Question 16.
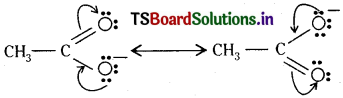
Write resonance structures of CH3COO and show the movement of electrons by curved arrows.
Answer:
First, write the structure and put unshared pairs of valence electrons on appropriate atoms. Then draw the arrows one at a time moving the electrons to get the other structures.
Question 17.
Write resonance structures of CH2=CH-CHO. Indicate relative stability of the contributing structures.
Answer:
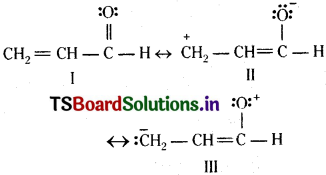
[I: Most stable, more number of covalent bonds, each carbon and oxygen atom has an octet and no separation of opposite
charge II:
negative charge on more electro-negative atom and positive charge on more electropositive atom; III: does not contribute as oxygen has positive charge and carbon has negative charge, hence least stable].
Question 18.
Explain why the following two structures, I and II cannot be the major contributors to the real structure of CH3COOCH3.
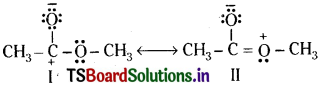
Answer:
The two structures are less important contributors as they involve charge separation. Additionally, structure I contains a carbon atom with an incomplete octet.
![]()
Question 19.
Explain why (CH3)3 C+ is more stable than and CH3C+ H2 is the least stable cation.
Answer:
Hyperconjugation interaction in (CH+ C is greater than in CH3 C H2 as the has nine C-H bonds. InC H3, vacant p orbital is perpendicular to the plane in which C-H bonds lie; hence cannot overlap with it. Thus, C H3 lacks hyper conjugative stability.
Question 20.
On complete combustion, 0.246 g of an organic compound gave 0.198g of carbon dioxide and 0.1014g of water. Determine the percentage composition of carbon and hydrogen in the compound.
Answer:
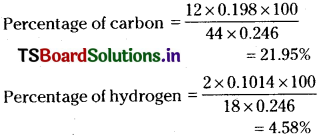
Question 21.
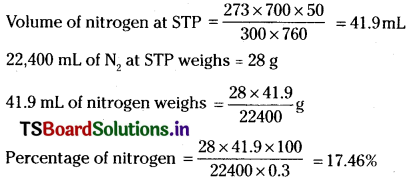
In Dumas’ method for estimation of nitrogen, 0.3 g of an organic compound gave 50 mL of nitrogen collected at 300 K temperature and 715 mm pressure. Calculate the percentage composition of nitrogen in the compound. (Aqueous tension at 300K = 15 mm)
Answer:
Volume of nitrogen collected at 300 K and 715 mm pressure is 50 mL
Actual pressure = 715 – 15 = 700mm
Question 22.
During estimation of nitrogen present in an organic compound by Kjeldahl’s method, the ammonia evolved from 0.5 g of the compound in Kjeldahl’s estimation of nitrogen, neutralized 10 mL of 1 M H2S04. Find out the percentage of nitrogen in the compound.
Answer:
1 M of 10 mL H,SO.=1M of 20 mL NH3
1000 mL of 1M ammonia contains 14 g nitrogen

Question 23.
In Carius method of estimation of halogen, 0.15 g of an organic compound gave 0.12 g of AgBr. Find out the percentage of bromine in the compound.
Answer:
Molar mass of AgBr = 108+80 = 188 g mol-1
188g AgBr contains 80 g bromine
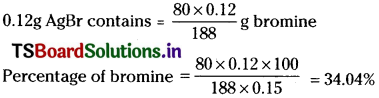
Question 24.
In sulphur estimation, 0.157 g of an organic compound gave 0.4813 g of barium sulphate. What is the percentage of sulphur in the compound?
Answer:
Molecular mass of BaS04 = 137 + 32 + 64 = 233 g
233 g BaSO4 contains 32 g sulphur

Question 25.
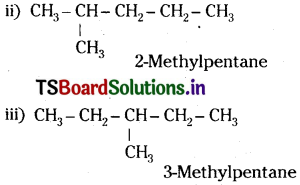
Write structures of different chain isomers of alkanes corresponding to the molecular formula C.H,,. Also write their IUPAC names.
Answer:

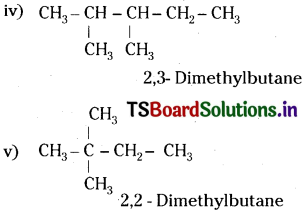
Question 26.
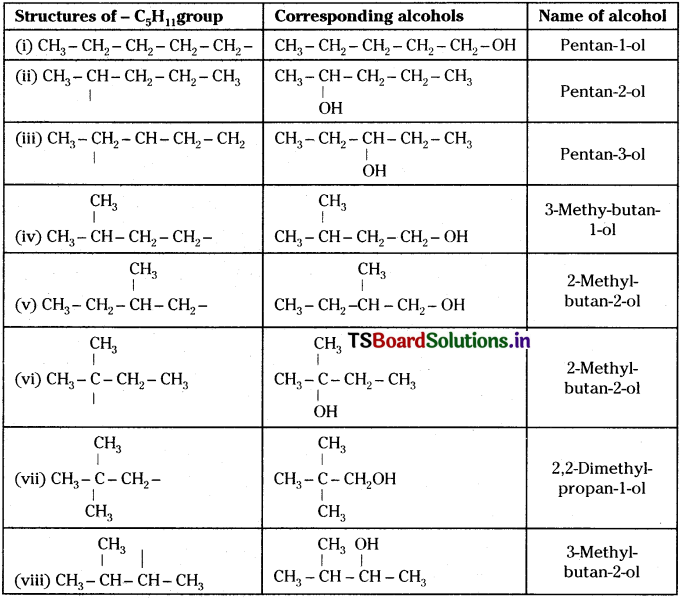
Write structures of different isomeric alkyl groups corresponding to the molecular formula C5H11. Write IUPAC names of alcohols obtained by attachment of -OH groups at different carbons of the chain.
Question 27.
Write IUPAC names of the following compounds:
(i) (CH3)3 C CH2C(CH3)3
(ii) (CH3)2 C(C2H5)2
(iii) tetra – tert-butylmethane
Answer:
(i) 2, 2, 4,4-Tetramethylpentane
(ii) 3, 3-Dimethylpentane
(iii) 3,3-Di-tert-butyl -2, 2, 4, 4 – tetramethylpentane
![]()
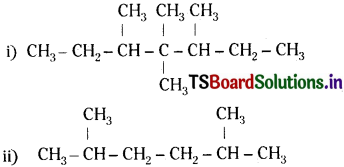
Question 28.
Write structural formulas of the following compounds:
(i) 3, 4, 4, 5-Tetramethylheptane
(ii) 2, 5-Dimethyhexane
Answer:
Question 29.
Write structures for each of the following compounds. Why are the given names incorrect? Write correct IUPAC names.
(i) 2-Ethylpentane
(ii) 5-Ethyl – 3-methylheptane
Answer:
i)

Longest chain is of six carbon atoms and not that of five. Hence, correct name is 3- Methylhexane.
ii)
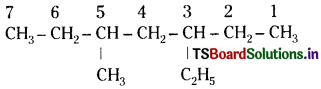
Numbering is to be started from the end which gives lower number to ethyl group. Hence, correct name is 3-ethyl-5- methyl- heptane.
Question 30.
Sodium salt of which acid will be needed for the preparation of propane? Write chemical equation for the reaction.
Answer:
Butanoic acid,
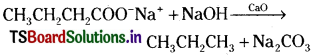
Question 31.
Write IUPAC names of the following compounds:

Answer:
(i) 2,8-Dimethyl-3, 6-decadiene;
(ii) 1, 3, 5, 7 Octatetraene;
(iii) 2-n-Propylpent-l-ene;
(iv) 4-Ethyl-2, 6-dimethyl-dec-4-ene;
![]()
Question 32.
Calculate number of sigma (σ) and pi (π) bonds in the above structures (i-iv).
Answer:
σ bonds : 33, π bonds : 2
σ bonds : 7, π bonds : 4
σ bonds : 23, π bond : 1
σ bonds : 41, π bond : 1
Question 33.
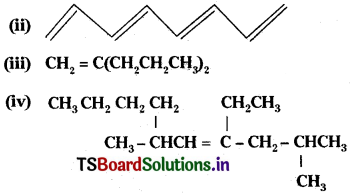
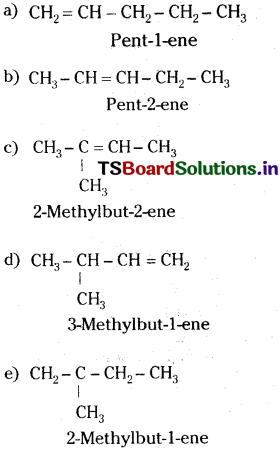
Write structures and IUPAC name of different structural isomers of alkenes corresponding to C5H10.
Answer:
Question 34.
Draw cis and trails isomers of the following compounds. Also write their IUPAC names:
(i) CHCl = CHCl (ii) C2H5CCH3 = CCH3C2H5
Answer:
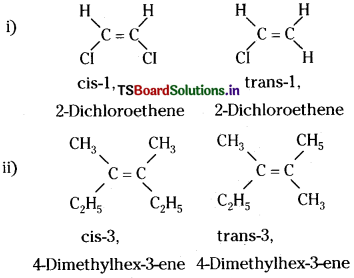
Question 35.
Which of the following compounds will showcistrans isomerism?
(i) (CH3)2C = CH-C2H5
ii) CH2 = CBr2
(iii) C6H5CH = CH – CH3
(iv) CH3CH = CClCH3
Answer:
(iii) and (iv). In structures (i) and (ii), two identical groups are attached to one of the doubly bonded carbon atom.
Question 36.
Write IUPAC names of the products obtained by addition reactions of HBr to hex-l-ene
(i) in the absence of peroxide and
(ii) in the presence of peroxide
Answer:
i) CH2 = CH – CH2- CH2-CH2- CH3+H – Br Hex-l-ene
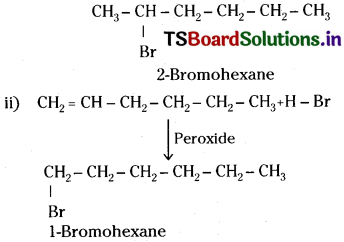
Question 37.
Write structures of different isomers corresponding to the 5th member of alkyne series. Also write IUPAC names of all the isomers. What type of isomerism is exhibited by different pairs of isomers?
Answer:
5th member of alkyne has the molecular formula C6H10. The possible isomers are:
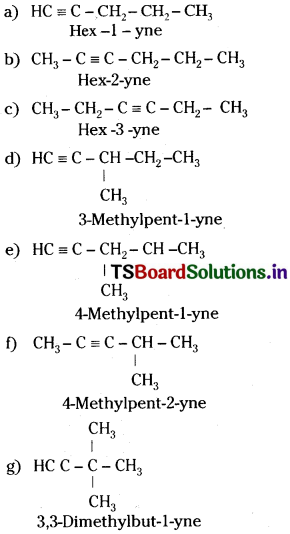
Position and chain isomerism shown by different pairs.
![]()
Question 38.
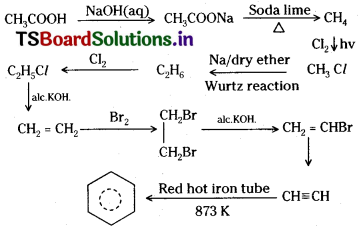
How will you convert ethanoic acid into benzene?
Answer: