Telangana TSBIE TS Inter 1st Year Chemistry Study Material 9th Lesson s-Block Elements Textbook Questions and Answers.
TS Inter 1st Year Chemistry Study Material 9th Lesson s-Block Elements
Very Short Answer Type Questions
Question 1.
Give reasons for the diagonal relationship observed in the periodic table.
Answer:
The diagonal relationship is due to the similarity in ionic sizes or similar polarising power.
![]()
Question 2.
Write completely the electronic configurations of K and Rb.
Answer:
At. no. of K = 19
Electronic configuration = 1s² 2s² 2p6 3s² 3p6 4s¹
At. no. of Rb = 37
Electronic configuration = 1s² 2s² 2p6 3s² 3p6 3d10 4s² 4p6 5s¹
Question 3.
Lithium salts are mostly hydrated. Why? [TS ’15]
Answer:
Li+ ion has small size and more charge density. So it has maximum degree of hydration. Due to this reason lithium salts are mostly hydrated, eg : LiCl. 2H2O
Question 4.
Which of the alkali metals shows abnormal density? What is the order of the variation of density among the IA group elements?
Answer:
Generally, density increases down the group. But potassium shows abnormal density. Its density is less than sodium. The order of densities of IA group elements is
Li < K < Na < Rb < Cs.
Question 5.
Lithium reacts with water less vigorously than sodium. Give your reasons. [Mar. ’18(TS)]
Answer:
Due to small size high IP value and very high hydration energy lithium reacts with water less vigorously.
![]()
Question 6.
Lithium Iodide is the most covalent among the alkali metal halides. Give the reasons.
Answer:
Li+ ion is small in size with more charge density among alkali metal ions. So according to Fajan’s rule it has more polarising power. T ion is bigger anion among halide ions and can undergo more polarisation. So the lithium iodide is more covalent among alkali metal halides.
Question 7.
In what respects lithium hydrogen carbonate differs from other alkali metal hydrogen carbonates?
Answer:
Lithium hydrogen carbonate cannot be prepared in solid state. It exists only in solution. The other alkali metal hydrogen carbonates can be prepared in solid state.
Question 8.
Write the complete electronic configurations of any two alkaline metals.
Answer:
1. Magnesium:
Atomic number = 12
Electronic configuration = 1s² 2s² 2p6 3s²
2. Calcium:
Atomic number = 20
Electronic configuration = 1s² 2s² 2p6 3s² 3p6 4s²
Question 9.
Tell about the variation of m. pts., and b.pts among the alkaline earth metals.
Answer:
The m. pts and b.pts of alkaline earth metals do not vary in a systematic manner. This is due to the difference in the structures of their metallic crystals.
Question 10.
What are the characteristic colours im-parted by the IIA elements?
Answer:
Be and Mg do not impart any colour in bunsen flame. They burn with white dazzling light. But other metals impart colour.
Ca- Brick red Sr – Crimson red Ba – Apple green
![]()
Question 11.
What happens when magnesium metal is parted by the II A elements? [Mar. ’18 (TS); (TS 15)]
Answer:
When magnesium metal is burnt in air it reacts with oxygen and nitrogen in the air forming oxide and nitride respectively.
2Mg + O2 → 2 MgO
3Mg + N2 → Mg3 N2
Question 12.
Lithium carbonate is not so stable to heat as the other alkali metal carbonates. Explain.
Answer:
Lithium ion being very small in size with more charge density polarises the bigger carbonate ion and distorts it. So it dissociates forming stable Li2O.
Li2 CO3 → Li2O + CO2
Question 13.
Write a balanced equation for the formation of ammoniated IIA metal ions from the metals in liquid ammonia.
Answer:
M + (x + y) NH3 → [M(NH3)x]+2 + 2[e(NH3)y]–
Question 14.
The fluorides of alkaline earth metals are relatively less soluble than their respective chlorides in water. Why?
Answer:
Fluoride ion is small in size. The lattice energy of ionic compounds is inversely proportional to the ionic sizes. So the fluorides of alkaline earth metals have high lattice energy. As size of other halide ions are large then lattice energy decreases. So fluorides of alkaline earth metals are relatively less soluble than their respective chlorides in water.
Question 15.
What happens when hydrated Mg (NO3)2 is heated? Give the balanced equation.
Answer:
When hydrated Mg (NO3)2 . 6H2O is heated first it converts into anhydrous compound and then decomposes.

Question 16.
Why does the solubility of alkaline earth metal hydroxides in water increase down the group?
Answer:
Down the group the lattice energies of alkali metal hydroxides decrease due to the increase in atomic size. Further down the group ionic character of these hydroxides increases due to increase in electropositive character. So the solubilities increase.
Question 17.
Why does the solubility of alkaline earth metal carbonates and sulphates in water decrease down the group?
Answer:
Down the group with increase in atomic size the lattice enthalpies and hydration enthalpies of carbonates and sulphates decrease but the decrease in hydration enthalpies is rapid than lattice enthalpies. So the solubilities of carbonates and sulphates decrease down the group.
![]()
Question 18.
Write the average composition of Portland cement.
Answer:
CaO 50 – 60%
SiO2 20 – 25%
Al2O3 5 – 10%
MgO 2 – 3%
Fe2O3 1 – 2%
SO3 1 – 2%
Question 19.
Why is gypsum added to cement? [TS Mar. ’19; (TS ’15)]
Answer:
Gypsum is added to cement to slow down the setting process.
Question 20.
Why are alkali metals not found in the free state in nature? [AP Mar. ’17, ’13]
Answer:
Because of high reactivity alkali metals do not occur in nature in the free state but occur only in the form of their chemical compounds.
Question 21.
Potassium carbonate cannot be prepared by Solvay process. Why? [AP Mar. ’19]
Answer:
Potassium bicarbonate is highly soluble in water. It cannot be precipitated by the ammonium hydrogen carbonate formed during Solvay process. So it cannot be prepared by Solvay process.
Question 22.
Describe the important uses of caustic soda. [AP ’16, ’15; May ’13]
Answer:
It is used in
- manufacture of soap, paper, artificial silk and number of chemicals.
- petroleum refining.
- purification of bauxite.
- textile industry for mercirising cotton.
- for the preparation of pure fats and oils.
- as a laboratory reagent.
Question 23.
Describe the important uses of sodium carbonate.
Answer:
It is used in.
- manufacture of soaps
- washing purposes
- removal of hardness of water
- fire extinguishers
Question 24.
Describe the important uses of quick lime. [IPE ’14]
Answer:
- It is used in the manufacture of cement,
- It is used in the manufacture of sodium carbonate and caustic soda.
- It is used in the purification of sugar and in the manufacture of dye stuffs.
- It is used in the production of refractories and as basic flux in metallurgy.
![]()
Question 25.
Draw the structures of i) BeCl2, (vapour) and ii) BeCl2 (solid).
Answer:
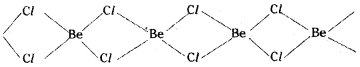
BeCl2 vapour contains dimer of BeCl2 which is linear, which decompose to linear monomer at high temperatures.
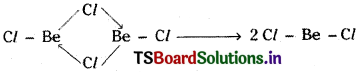
Solid BeCl2 contains polymeric structure.
Question 26.
Describe the importance of Plaster of Paris.
Answer:
It has a remarkable property of setting with water. So it is used in the building industry. It is used in plastering the fractured bones. It is also used in dentistry and in making casts of statues and busts.
Question 27.
Which of the alkaline earth metal carbonates is thermally the most stable? Why?
Answer:
BaCO3 is the most stable carbonate of alkaline earth metal carbonates. The bigger cation Ba2+ has less polarising power, cannot distort the carbonate ion. So stability is more.
Question 28.
Write balanced equations for the reactions between
i) Na2O2 and water; ii) K2O and water.
Answer:
i) Na2O2 + 2H2O → 2NaOH + H2O2 (in dilute solution)
2Na2O2 + 2H2O → 4NaOH + O2 (in concentrated solution)
ii) K2O + H2O → 2KOH
Short Answer Questions
Question 1.
Alkali metals and their salts impart characteristic colours to an oxidizing flame. Explain the reason.
Answer:
The alkali metals and their salts impart characteristic colour to an oxidising flame. This is because the heat from the flame excites the outermost orbital electron to a high energy level. When excited electrons come back to the ground state, they emit the absorbed energy in the form of light in visible region.
Lithium – Crimson red
Sodium – Yellow
Potassium – Lilac or pale violet
Rubidium – Red violet
Caesium – Blue violet
Question 2.
What makes caesium and potassium useful as electrodes in photoelectric cells?
Answer:
The ionisation energies of caesium and potassium are very small. When light falls on the surface of metals, the energy present in them is absorbed by the atoms on the surface of metal. The energy of light is sufficient to make an atom to lose electron. This property makes caesium and potassium useful as electrodes in photoelectric cells.
Question 3.
Write a short note on the reactivity of alkali metals towards air.
Answer:
The alkali metals When exposed to air react with oxygen, moisture and carbon dioxide forming oxide, hydroxide and carbonate. So they immediately tarnish in air.
eg: 4Na + O2 → 2Na2O
Na2O + H2O → 2NaOH
2NaOH + CO2 → Na2CO3 + H2O
Alkali metals burn in air forming oxide. Lithium forms only monoxide.
4Li + O2 → 2 Li2O
Sodium forms monoxide in limited supply of air but forms peroxide in excess of oxygen.
4Na + O2 → 2 Na2O2
2Na + O2 → Na2O2
Other alkali metals form superoxides
M + O2 (excess) → M O2(M = K, Rb, Cs)
Question 4.
Give any two uses for each of the following metals.
i) Lithium ii) Sodium
Answer:
i) Lithium:
a) Lithium metal is used in preparation of alloys.
Eg : White metal is an alloy of lithium and lead used in making bearings for motor engines.
The alloy of lithium-magnesium is used to make armour plates.
b) Lithium is used in thermonuclear reactions.
c) Lithium is used in making electrochemical cells.
ii) Sodium:
a) Sodium-lead alloy is used in making tetraethyl lead an anti knocking agent in petrol.
b) Liquid sodium metal is used as coolant in nuclear reactors.
![]()
Question 5.
Give an account of the properties of washing soda.
Answer:
Chemical properties of Na2CO3 :
(1) Action with acids: CO2 gas is liberated.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
(2) Action with non-metals and their oxides: Sodium carbonate reacts with a mixture of S and SO2 and form Hypo (sodium thiosulphate)
Na2CO3 + SO2 + S → Na2S2O3 + CO2
(3) Action with COz : An aq. solution of sodium carbonate when saturated with CO2 gives ppt. of sodium bicarbonate.
Na2CO3 + H2O + CO2 → 2NaHCO3
(4) Action with silica : When fused with SiO2, water glass is formed.
Na2CO3 + SiO2 → Na2SiO3 + CO2
(5) Action with compounds : Insoluble metal carbonates are formed.
MgCl2 + Na2CO3 → MgCO3 + 2NaCl
ZnSO4 + Na2CO3 → ZnCO3 + Na2SO4
Aqueous Na2CO3 solution is basic in nature. So methyl orange produces yellow colour in that solution.
Question 6.
Mention some uses of sodium carbonate.
Answer:
Sodium carbonate is used
- in the manufacture of glass, water glass, caustic soda, paper, dyes.
- to remove hardness of water.
- as a reagent in the laboratory in qualitative and quantitative analysis.
- in laundries for washing purposes.
- in the manufacture of indigo.
- in petroleum industry.
Question 7.
How do you obtain pure sodium chloride from a crude sample?
Answer:
Crude sodium chloride, generally obtained by crystallisation of brine solution, contains sodium sulphate, calcium sulphate, calcium chloride and magnesium chloride as impurities. To get pure sodium chloride first the crude sodium chloride is dissolved in minimum amount of water and filtered to remove insoluble impurities.
Now into the saturated solution of sodium chloride hydrogen chloride gas is passed. Then pure sodium chloride crystallises. The other compounds which are present in small amounts such as MgCl2 and CaCl2 remain in solution.
Question 8.
What do you know about Castner – Kellner process? Write the principle involved in it.
Answer:
This process is also called Mercury Cathode process.
Principle :
Brine solution is electrolysed using mercury as cathode. Chlorine gas evolved at anode escapes out through the outlet. At the same time sodium amalgam is formed at cathode. This sodium amalgam reacts with water and gives NaOH along with the evolution of H2 gas.
2NaCl → 2Na+ + 2Cl– (ionization)
At Mercury Cathode:
2Na+ + 2e– + Hg → Na2Hg
At Graphite Anodes:
2Cl– → Cl2 ↑ + 2e–
In the Central Compartment:
At anode : Na2Hg → 2Na+ + 2e– + Hg
At cathode: 2H2O + 2e– → 2OH– + H2↑
2Na+ + 2OH– → 2 NaOH
Process :
The Castner’s cell consists of a rectangular iron tank, divided into 3 compartments by two slate partitions as shown in the diagram. The partitions will be upto the bottom but they do not touch the bottom. The bottom of the tank is covered with mercury. In the middle compartment mercury acts as anode and in the outer compartments it acts as cathode. Brine solution is taken in the outer compartments. Graphite rods, projected into the brine solution act as anode. In the middle compartment dil. NaOH solution is taken. A bunch of iron rods suspended in the middle compartment acts as cathode.
The cathode and anode in the outer and middle compartments are connected by means of wires to the terminals of a battery. Then the above reactions occur and a concentrated solution of NaOH is formed in the middle compartment. It is evaporated in iron pans, cooled and cast into moulds.
![]()
Question 9.
Write a few applications of caustic soda. [AP ’15; May ’13]
Answer:
It is used in
- manufacture of soap, paper, artificial silk and number of chemicals.
- petroleum refining.
- purification of bauxite.
- textile industry for mercirising cotton.
- for the preparation of pure fats and oils.
- as a laboratory reagent.
Question 10.
Give an account of the biological importance of Na+ and K+ ions. [Mar. ’18 (AP)]
Answer:
Biological importance of sodium:
(1) Na+ ions help in the transmission of nerve signals. (2) Na+ ions help in regulating the flow of water across the cell membranes. (3) Na+ ions help in transporting sugars and amino acids into the cells.
Biological importance of potassium :
(1) K+ ions help in activating many enzymes. (2) K+ ions participate in oxidising glucose to produce ATP. (3) They also help in transmitting nerve signals.
Question 11.
Mention the important uses of Mg metal.
Answer:
- Magnesium forms alloys with aluminium, zinc, manganese and tin.
Mg – Al alloys are light in mass and are used in aircraft construction. - Mg powder is used in flash powders and signals.
- A suspension of magnesium hydroxide in water called milk of magnesia is used as antacid in medicine.
- Magnesium carbonate is an ingredient of toothpaste.
Question 12.
Show that Be(OH)2 is amphoteric in nature.
Answer:
Beryllium hydroxide can react with both acids and bases.
Be(OH)2 + 2HCl → BeCl2 + 2H2O
Be(OH)2 + 2NaOH → Na2 BeO2 + 2H2O
The reaction of Be(OH)2 with hydrochloric acid shows the basic nature, while the reaction with sodium hydroxide shows the acidic nature. So Be(OH)2 is amphoteric.
Question 13.
Write a note on the anomalous behavior of beryllium.
Answer:
Anomalous characters of Beryllium :
(a) Compounds of Be are predominantly covalent.
(b) It is not easily affected by air and does not decompose water at ordinary tem-perature.
(c) It is an amphoteric metal. It dissolves in alkali solutions forming beryllates.
(d) BeSO4 is soluble in water whereas the sulphates of Ca, Sr and Ba are insoluble.
(e) Be and its salts do not respond to Flame Test while Ca, Sr and Ba give characteristic flame colours.
(f) Beryllium forms many complexes, while heavier elements do not form complexes easily.
(g) Be has a maximum covalency of 4, while others can have a maximum covalency of six.
Question 14.
Be shows diagonal relationship with Al. Discuss.
Answer:
The ionic radius of Be2+ is nearly the same as that of Al3+ ion. Hence beryllium resembles aluminium in some properties.
- Both beryllium and aluminium are not readily attacked by acids, because of the presence of an oxide film on the surface of the metal.
- Hydroxides of both beryllium and aluminium are amphoteric and dissolve in both acids and bases. With bases Be(OH)2 forms beryl late ion [Be(OH)4]2- while Al(OH)3 forms aluminate ion [Al(OH)4]–.
- The chlorides of both beryllium and aluminium have chloride bridge bonds in vapour phase. Both the chlorides are soluble in organic solvents and are strong Lewis acids. These are used as Friedel Craft catalysts.
- Beryllium and aluminium ions have strong tendency to form complexes BeF-24, AlF3-6.
Question 15.
What is Plaster of Paris? Write a short note on it. [Mar. ’19, ’18 (AP) (AP Mar. 17. 15, 16; TS 16)]
Answer:
Gypsum on heating to a temp, of 120° – 130°C gives a semi hydrate. It is known as Plaster of Paris.

When a water paste of plaster of paris is allowed to stand for sometime it sets to hard mass. This is called setting of Plaster of Paris. This is an exothermic process and involves two stages. In the first stage, plaster of paris is converted into orthorhombic dihydrate. In the second stage, the orthorhombic dihydrate is converted into monoclinic dihydrate.
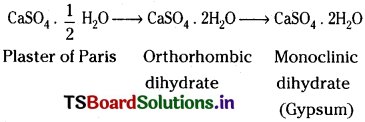
It is used (1) in making casts for statues, toys, etc. (2) in surgical bandages for bone fracture (3) in making white chalks.
![]()
Question 16.
In what ways lithium shows similarities to magnesium in its chemical behavior?
Answer:
In the periodic table the first element of a group in the 2nd period shows similar properties with the second element of the next group in the third period. This relationship is known as diagonal relationship.
Li shows similarity with Mg in the following respects.
(a) Lithium is slow to react with water. Mg decomposes water in hot condition.
(b) Both lithium and magnesium give monoxides only.
(c) LiCl is deliques’cent like MgCl2.
(d) Halides of Lithium and Magnesium are soluble in organic solvents.
(e) Both Li+ and Mg+2 ions are highly hydrated.
(f) The carbonates, phosphates and fluo-rides of both Lithium and Magnesium are sparingly soluble in water.
(g) Lithium alkyls are chemically similar to Grignard reagents in organic synthesis.
Question 17.
When an alkali metal dissolves in liquid ammonia the solution can acquire different colours. Explain the reasons for this type of colour change.
Answer:
The alkali metals dissolve in liquid ammonia giving deep blue solutions which are conducting in nature.
M + (x + y) NH3 → [M(NH3)x]+ + [e (NH3)y]–
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution. The solutions are paramagnetic and on standing slowly liberate hydrogen resulting in the formation of amide.
If the concetration of alkali metal in ammonia increases the ammoniated electrons start pairing and the colour changes to bronze colour.
Question 18.
What happens when
i) Sodium metal is dropped in water?
ii) Sodium metal is heated in a free supply of air?
iii) Sodium peroxide dissolves in water?
Answer:
1) When sodium metal is dropped in water vigorous reaction takes place liberating hydrogen. Here sodium metal is oxidised while water is reduced.
2Na + 2H2O → 2NaOH + H2
The reaction is highly exothermic and the hydrogen catches fire. The solution becomes basic.
2) When sodium metal is heated in a free supply of air sodium peroxide is formed.
2Na + O2 → Na2O2
3) Sodium peroxide when dissolved in water in dilute solution gives hydrogen peroxide but in concentrated solution gives oxygen.
Na2O2 + 2H2O → 2NaOH + H2O2 (in dilute solution)
2Na2O2 + 2H2O → 4NaOH + O2 (in concentrated solution)
Question 19.
State as to why
i) An aqueous solution of Na2COs is alkaline.
ii) Alkali metals are prepared by the electrolysis of their fused chlorides?
Answer:
i) Sodium carbonate when dissolved in water react with water producing alkaline solution.
Na2CO3 + 2H2O → 2Na+ 2OH– + H2CO3
Sodium carbonate hydrolyses in water producing strong base NaOH and weak carbonic acid H2CO3. NaOH being strong electrolyte ionises completely but H2CO3 does not ionise. So the solution is alkaline, ii) Alkali metals themselves are strong reducing agents. So a reducing agent stronger than alkali metal is not available. Hence they cannot be extracted by chemical reduction method.
When aqueous solutions of alkali metal salts are electrolysed hydrogen gas will be liberated at cathode instead of alkali metal. This is because the discharge potential of H+ is less than Na+.
Hence alkali metals are prepared by the electrolysis of fused chlorides. To decrease the melting points of the alkali metal halides they are mixed with some other compounds.
Question 20.
How would you explain the following observations?
i) BeO is almost insoluble but BeSO4 is soluble in water?
ii) BaO is soluble but BaSO4 is insoluble in water?
Answer:
i) When small catibn combines with small anion lattice energy is very high. So the lattice energy of BeO is more than its hydration energy. Hence BeO is insoluble.
When small beryllium ion combines with large anion the lattice energy decreases more rapidly than the decrease in hydration energy. Here lattice energy is less than hydration energy. Hence BeSO4 is soluble.
ii) When small anion (O-2) combines with large cation (Ba2+) lattice energy is less. But at the same time the hydration energy exceeds the lattice energy. So BaO is soluble in water.
In the case of BaSO4 both cation and anion are large. So the lattice energy is less. But due to the bigger size of Ba2+ and SO2-4 their hydration energies are also less. The sum of hydration energies of Ba2+ and SO2-4 is less than the lattice energy of BaSO4. So BaSO4 is insoluble in water.
Long Answer Questions
Question 1.
Justify the inclusion of alkali metals in the same group of the periodic table with reference to the following, i) Electronic configuration ii) Reducing nature iii) Oxides and hydroxides.
Answer:
i) The electronic configurations of alkali metals are as follows.
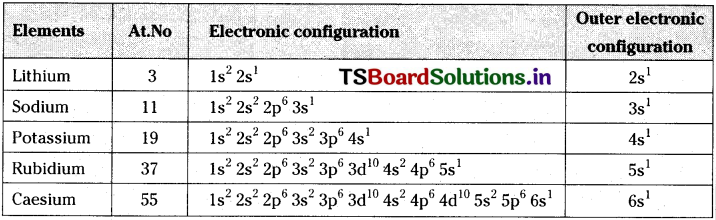
All these elements have same outer electronic configuration. The modern periodic table is developed basing on electronic configuration. Since all these elements having same outer electronic configuration their inclusion in the same group is justified.
ii) Reducing nature:
Alkali metals have bigger atomic sizes and have low I.P values. So they have a tendency to lose electron and thus they act as strong reducing agents. Among alkali metals lithium is the strongest reducing agent while sodium is the weaker reducing agent. The large hydration energy of small Li+ ion makes lithium the strongest reducing agent.
iii) Oxides and Hydroxides :
The oxides and hydroxides of all the alkali metals are strongly alkaline. Because of high electropositive character of alkali metals, their oxides and hydroxides are strongly basic.
Because of these similarities in these properties their inclusion in the same group is justified.
![]()
Question 2.
Write an essay on the differences between lithium and other alkali metals.
Answer:
The anomalous behaviour of lithium is due to the: (i) exceptionally small size of its atom and ion, and (ii) high polarising power (i.e., charge / radius ratio). As a result, there is increased covalent character of lithium compounds which is responsible for their solubility in organic solvents.
Some of the abnormal properties of lithium are given below.
- Lithium is much harder.
- Lithium is least reactive but the strongest reducing agent among all the alkali metals. On combustion in air it forms mainly monoxide. Li2O and the nitride. Li3N, unlike other alkali metals.
- LiCl is deliquescent and crystallises as a hydrate, LiCl.2H2O whereas other alkali metal chlorides do not form hydrates.
- Lithium hydrogencarbonate is not obtained in the solid form while all other elements form solid hydrogencarbonates.
- Lithium unlike other alkali metals forms no ethynide on reaction with ethyne.
- Lithium nitrate when heated gives lithium oxide, Li2O, whereas other alkali metal nitrates decompose to give the correspon-ding nitrites.
4LiNO3 → 2 Li2O + 4 NO2 + O2
2NaNO3 → 2NaNO2 + O2 - LiF and Li2O are comparatively much less soluble in water than the corresponding compounds of other alkali metals.
Question 3.
Discuss the preparation and properties of sodium carbonate.
Answer:
i) Sodium carbonate is prepared by Ammonia soda (or) Solvay process.
Raw materials:
The raw materials required are 1) Brine solution (saturated) 2) Ammonia and 3) Lime stone.
Principle:
Brine solution is saturated with Ammonia and CO2 gas is passed through it. Then sodium bicarbonate is formed.
NH3 + H2O + CO2 → NH4HCO3
NH4HCO3 + NaCl → NaHCO3 + NH4Cl
The sodium bicarbonate thus formed on heating decomposes to give sodium carbonate.
2NaHCO3 → Na2CO3 + H2O + CO2
Process:
1) Saturation of Brine with ammonia :
Ammonia absorber is filled with brine solution and saturated with ammonia gas containing a little amount of CO2. Then the calcium and magnesium impurities present in the brine, precipitate as their carbonates and hydroxides.
2NH3 + H2O + CO2 → (NH4)2 CO3
MgCl2 + (NH4)2CO3 → MgCO3 + 2NH4Cl
The precipitates are filtered in “Filter press” and the filtrate is cooled and sent to Carbonation Tower.
2) Carbonation of ammoniacal Brine :
This process is carried out in Carbonation tower. It is a tall cylindrical tower containing perforated plates arranged one above the other Ammonical brine solution is dropped from upper half and CO2 gas is passed from the lower part. Then both of them react together forming sodium bicar-bonate.
NH3 + H2O + CO2 → NH4HCO3
NaCl + NH4HCO3 → NaHCO3 + NH4Cl
3) Filtration :
The dense liquid from the carbonation tower is sent into rotary vacuum filter and filtered.
4) Fusion of sodium bicarbonate:
The NaHCO3 obtained in the Rotary filter is heated to high temperatures. Then NaHCO3 decomposes to give Na2CO3.
![]()
5) Recovery of Ammonia :
The filtrate from the Vacuum filter is pumped into ammonia recovery tower, mixed with Ca(OH)2 and heated with steam. Then NH3 gas is liberated which is sent back to saturation tower.
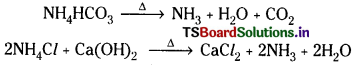
Note : The milk of lime required for the regeneration of NH3 is obtained from the Lime Kiln by heating lime stone.

ii) Chemical properties of Na2CO3 :
Chemical properties of Na2CO3 :
(1) Action with acids: CO2 gas is liberated.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
(2) Action with non-metals and their oxides: Sodium carbonate reacts with a mixture of S and SO2 and form Hypo (sodium thiosulphate)
Na2CO3 + SO2 + S → Na2S2O3 + CO2
(3) Action with CO2 : An aq. solution of sodium carbonate when saturated with CO2 gives ppt. of sodium bicarbonate.
Na2CO3 + H2O + CO2 → 2NaHCO3
(4) Action with silica : When fused with SiO2, water glass is formed.
Na2CO3 + SiO2 → Na2SiO3 + CO2
(5) Action with compounds : Insoluble metal carbonates are formed.
MgCl2 + Na2CO3 → MgCO3 + 2NaCl
ZnSO4 + Na2CO3 → ZnCO3 + Na2SO4
Aqueous Na2CO3 solution is basic in nature. So methyl orange produces yellow colour in that solution.
![]()
Question 4.
Discuss the similarities between alkaline earth metals and gradation in the following aspects:
i) Electronic configuration; ii) Hydration enthalpies; iii) Nature of the oxides and hydroxides.
Answer:
i) Electronic configuration of alkaline earth metals :
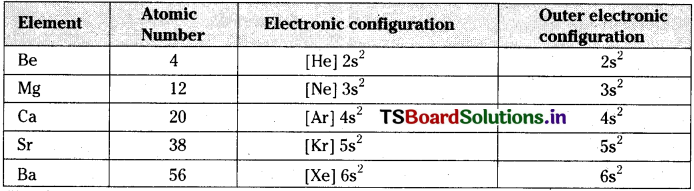
All the alkaline earth metals have same outer electrons but gradually the atomic size increases due to increase in the number of orbits.
ii) Hydration enthalpies :
Alkali metal ions (M2+) have more charge and small size. So they have high hydration enthalpies. But with the increase in ionic size the attraction towards water molecules decreases. So hydration enthalpies of alkaline earth metal ions decreases gradually down the group.
iii) Nature of oxides and hydroxides :
The oxides and hydroxides of alkaline earth metals are strongly alkaline in nature. BeO and Be(OH)2 are amphoteric. The oxides of other elements are ionic and basic in nature. The oxides react with water forming hydroxides.
MO + H2O → M(OH)2
The solubility, thermal stability and the basic character of these oxides and hydroxides increase with increasing atomic number.
Question 5.
Discuss on : i) Carbonates ; ii) Sulphates and iii) Nitrates of alkaline earth metals.
Answer:
i) Carbonates:
Carbonates of alkaline earth metals are insoluble in water and can be precipitated by addition of sodium or ammonium carbonate solution to the solutions of soluble compounds of these metals.
The solubility of the carbonates decre-ases down the group. Thermal stability of these carbonates increases down the group. So their decomposition temperatures increase down the group.
ii) Sulphates:
Sulphates are white solids. Their solubility decreases down the group. Thermal stability increases down the group. BeSO4 and MgSO4 are soluble. The greater hydration enthalpies of Be2+ and Mg2+ ions overcome the lattice enthalpy factor and therefore their sulphates are soluble.
iii) Nitrates:
These can be prepared by dissolving their carbonates in dilute nitric acid. These crystallise from their solution as their hydrates. Barium nitrate crystallise as anhydrous salt. This is because of the decrease in the hydration enthalpies. All these nitrates decompose on heating.
2M (NO3)2 → 2MO + 4NO2 + O2 (M = Be, Mg, Ca, Sr, Ba)
Question 6.
What are the common physical and chemical features of alkali metals?
Answer:
Physical properties:
- All the alkali metals are soft metals with low m.pts and b.pts
- In each period the alkali metals have large atomic sizes.
- All the alkali metals exhibit only one oxidation state + 1.
- All the alkali metals exhibit flame colours.
- Alkali metals have low IP and have tendency to lose electrons. So they act as strong reducing agents.
Chemical Properties:
- All the alkali metals react with oxygen in air forming oxides.
- All the alkali metals react with water liberating hydrogen. The reactivity of alkali metals increases down the group.
- All the alkali metals react with hydrogen forming ionie hydrides.
- All the alkali metals react with halogen forming similar halides of the type Mx.
- The oxides and hydroxides of the alkali metals are strongly alkaline.
- All the alkali metals dissolve in ammonia forming blue coloured solutions due to the presence of ammoniated electrons. These solutions are good reducing agents, good conductors of electricity and paramagnetic.
![]()
Question 7.
Discuss the general characteristics and – gradation in properties of alkaline earth metals.
Answer:
- Atomic size increases from top to bottom in the group due to increase in the number of orbits.
- Densities increase from top to bottom in the group but Ca is less denser than Mg.
- M.pts and b.pts do not vary regularly.
- Ionisation enthalpies decrease from top to bottom.
- Hydration enthalpies decrease from top to bottom in the group.
- Reactivity towards air and water increases from top to bottom in the group. All these elements burn in air forming metal oxides and metal nitrides. Be and Mg do not react with water but other elements react with water and the reactivity increases from Ca to Ba.
- All these elements react with halogens forming halides.
- All these elements except beryllium react with hydrogen directly forming ionic hydrides.
- These elements readily react with acids liberating hydrogen.
- Alkaline earth metals are good reducing agents and their reduction power increases from top to bottom.
Question 8.
Discuss the various reactions that occur in the Solvay process. [AP ’16]
Answer:
i) Sodium carbonate is prepared by Ammonia soda (or) Solvay process.
Raw materials:
The raw materials required are 1) Brine solution (saturated) 2) Ammonia and 3) Lime stone.
Principle:
Brine solution is saturated with Ammonia and CO2 gas is passed through it. Then sodium bicarbonate is formed.
NH3 + H2O + CO2 → NH4HCO3
NH4HCO3 + NaCl → NaHCO3 + NH4Cl
The sodium bicarbonate thus formed on heating decomposes to give sodium carbonate.
2NaHCO3 → Na2CO3 + H2O + CO2
Process:
1) Saturation of Brine with ammonia :
Ammonia absorber is filled with brine solution and saturated with ammonia gas containing a little amount of CO2. Then the calcium and magnesium impurities present in the brine, precipitate as their carbonates and hydroxides.
2NH3 + H2O + CO2 → (NH4)2 CO3
MgCl2 + (NH4)2CO3 → MgCO3 + 2NH4Cl
The precipitates are filtered in “Filter press” and the filtrate is cooled and sent to Carbonation Tower.
2) Carbonation of ammoniacal Brine :
This process is carried out in Carbonation tower. It is a tall cylindrical tower containing perforated plates arranged one above the other Ammonical brine solution is dropped from upper half and CO2 gas is passed from the lower part. Then both of them react together forming sodium bicar-bonate.
NH3 + H2O + CO2 → NH4HCO3
NaCl + NH4HCO3 → NaHCO3 + NH4Cl
3) Filtration :
The dense liquid from the carbonation tower is sent into rotary vacuum filter and filtered.
4) Fusion of sodium bicarbonate:
The NaHCO3 obtained in the Rotary filter is heated to high temperatures. Then NaHCO3 decomposes to give Na2CO3.
![]()
5) Recovery of Ammonia :
The filtrate from the Vacuum filter is pumped into ammonia recovery tower, mixed with Ca(OH)2 and heated with steam. Then NH3 gas is liberated which is sent back to saturation tower.

Note : The milk of lime required for the regeneration of NH3 is obtained from the Lime Kiln by heating lime stone.

ii) Chemical properties of Na2CO3 :
Chemical properties of Na2CO3 :
(1) Action with acids: CO2 gas is liberated.
Na2CO3 + 2HCl → 2NaCl + H2O + CO2
(2) Action with non-metals and their oxides: Sodium carbonate reacts with a mixture of S and SO2 and form Hypo (sodium thiosulphate)
Na2CO3 + SO2 + S → Na2S2O3 + CO2
(3) Action with CO2 : An aq. solution of sodium carbonate when saturated with CO2 gives ppt. of sodium bicarbonate.
Na2CO3 + H2O + CO2 → 2NaHCO3
(4) Action with silica : When fused with SiO2, water glass is formed.
Na2CO3 + SiO2 → Na2SiO3 + CO2
(5) Action with compounds : Insoluble metal carbonates are formed.
MgCl2 + Na2CO3 → MgCO3 + 2NaCl
ZnSO4 + Na2CO3 → ZnCO3 + Na2SO4
Aqueous Na2CO3 solution is basic in nature. So methyl orange produces yellow colour in that solution.
Question 9.
Starting with sodium chloride how would you proceed to prepare
i)Sodium metal; ii) Sodium hydroxide; iii) Sodium peroxide; iv) Sodium carbonate.
Answer:
1) Sodium metal preparation:
When molten sodium chloride is electrolysed sodium metal is formed. To decrease the melting point of sodium chloride it is mixed with KCl and CaCl2.

2) Sodium hydroxide :
Electrolysis of aqueous sodium chloride either in Nelson’s cell or in Castner kellner cell gives sodium hydroxide.
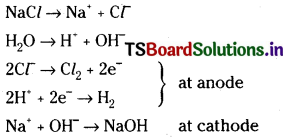
3) Sodium peroxide :
First sodium metal is prepared from sodium chloride as above. Then it is burnt in excess of O2 to produce sodium peroxide.
2Na + O2 → Na2O2
4) Sodium carbonate:
Brine is saturated with ammonia. Then CO2 is passed into the solution. Sodium bicarbonate is formed due to the following reactions.
NH3 + H2O + CO2 → NH4 HCO3
NaCl + NH4 HCO3 → NaHCO3 + NH4Cl
The sodium bicarbonate on calcination gives sodium carbonate.
2NaHCO3 → Na2CO3 + H2O + CO2
![]()
Question 10.
What happens when
i) Magnesium is burnt in air? [Mar. ’18 (TS)]
ii) Quick lime is heated with silica?
iii) Chlorine reacts with slaked lime?
iv) Calcium nitrate is strongly heated?
Answer:
i) When magnesium is burnt in air it reacts with oxygen and nitrogen in air forming magnesium oxide and magnesium nitride.
2Mg + O2 → 2MgO
3Mg + N2 → Mg2N2
ii) Quick lime is CaO. When heated with silica it forms calcium silicate. This is because CaO is basic and silica is acidic.
CaO + SiO2 → CaSiO3
iii) Chlorine reacts with slaked lime: When dry chlorine gas is passed over dry slaked lime bleaching powder is formed.
2Ca(OH)2 + 2Cl2 → CaCl2 + Ca(OCl)2 + 2H2O
iv) On heating calcium nitrate it decomposes giving CaO, NO2 and O2
2 CaCNO3)2 → 2CaO + 4NO2 + O2
Question 11.
Explain the significance of sodium, potassium, magnesium and calcium in biological fluids. [TS ’16; Mar. ’13]
Answer:
The ions of alkali metals balance the charges associated with -vely charged organic molecules present in the cells. The ions help in maintaining the osmotic pressure in the cell. The presence of Na+ and K+ ions inside and outside the cell produces an electrical potential across the cell membrane. The presence of Na+ ions is associated with the movement of glucose into cells. The excess Na+ ions entering the cell are expelled in the pumping out process. The migration of amino acids is similar.
The K+ ions are essential for the meta-bolism of glucose inside the cell, the synthesis of proteins and the activation of certain enzymes.
Mg+2 ions are concentrated in animal cells. Enzymes like “Phosphohydrolases” and “Phosphotransferases” contain Mg+2 ions. These enzymes participate in ATP reactions and release energy. Mg+2 forms a complex with ATP. Mg+2 is a constituent of chlorophyll.
Ca+2 is present in bones and teeth as apatite. Enamel on teeth is Fluorapatite. Ca+2 ions are necessary for blood clotting. These are necessary to maintain regular heart beating. These are also necessary for muscle contraction.
Question 12.
Write a few lines about cement.
Answer:
Cement was discovered by Joseph Aspidin. It appears like natural limestone available at Portland in England. So it is also called as Portland cement.
It is prepared by heating a mixture of limestone and clay. The clay contains Si02 along with oxides of aluminium, iron and magnesium. The CaO obtained from lime-stone combines with these oxides and con-verts into dicalcium silicate, tricalcium silicate and tricalcium aluminate. The hard mass containing these compounds is called clinker. It is powdered, mixed with little gypsum to slow down the setting process and pawed in bags.
The average composition of cement is
CaO – 50 to 60%
SiO2 – 20 to 25%
Al2O3 – 5 to 10%
MgO – 2 to 3%
Fe2O3 -1 to 2%
SO3 -1 to 2%
For good quality cement the ratio of silica to alumina should be 2.5 and 4 and the ratio of lime to the total oxides of silicon (SiO2), aluminium (Al2O3) and iron (Fe2O3) should be nearly 2.
When cement is wetted with water it sets into hard mass. This is known as sett-ing of cement. Setting of cement is due to hydration of the molecules of the constituents and their rearrangement.
Cement is used in concrete, reinforced concrete, plastering, and in the construction of bridges, dams, and buildings.
![]()
Question 13.
Uses of Mg.
Answer:
- Milk of Magnesium is used as an antacid.
- Mg powder is used in flash bulbs.
- Mg and Al form alloys. They are used in making aeroplane spare parts.