Telangana TSBIE TS Inter 1st Year Chemistry Study Material 8th Lesson Hydrogen and its Compounds Textbook Questions and Answers.
TS Inter 1st Year Chemistry Study Material 8th Lesson Hydrogen and its Compounds
Very Short Answer Type Questions
Question 1.
The three isotopes of hydrogen differ in their rates of reaction. Give the reasons.
Answer:
Due to the difference in the bond dissociation enthalpies of the H2, D2 and T2 molecules differ in their rates of reaction. The reactivity order is H2 > D2 > T2.
Question 2.
Why is dihydrogen used in weldipg of high melting metals?
Answer:
Atomic hydrogen and oxy-hydrogen torches generate very high temperature of 4000K. So dihydrogen is used in welding of high-melting metals.
Question 3.
Describe one method of producing high-purity hydrogen.
Answer:
High purity (> 99.95%) dihydrogen can be obtained by electrolysing warm aqueous barium hydroxide solution using nickel electrodes.
Question 4.
Explain the term “SYNGAS”.
Answer:
The mixture of CO and H2 is called as water gas or SYNGAS because it is used in the synthesis of methanol and a number of hydrocarbons.
Question 5.
What is meant by coal gasification? Explain with relevant, balanced equation.
Answer:
The process of producing SYNGAS from coal is called coal gasification.
![]()
![]()
Question 6.
Define the term Hydride. How many categories of hydrides are known? Name them.
Answer:
The binary compounds of hydrogen with other elements are called hydrides.
The hydrides are classified into three categories.
- Ionic or saline or salt like hydrides.
- Covalent or molecular hydrides.
- Metallic or non-stoichiometric hydrides.
Question 7.
The unusual property of water in condensed phase leads to its high heat of vapourization. What is that property?
Answer:
The unusual property of water in condensed phase that leading to its high heat of vapourisation is due to the presence of extensive hydrogen bonding between water molecules.
Question 8.
During photosynthesis, water is oxidized to O2. Which element is reduced?
Answer:
The photosynthesis reaction is
6 CO2 + 6 H2O → C6 H12 O6 + 6 O2
The oxidation number of carbon in CO2 is + 4 but in carbohydrate formed the oxidation number of carbon is O. Thus carbon is reduced.
Question 9.
What do you mean by autoprotolysis? Give the equation to represent the auto-protolysis of water.
Answer:
Self – ionisation of water is known as autoprotolysis.
![]()
Because of this property, water acts as Bronsted acid when dissolved in alkalis and also acts as Bronsted base when dissolved in acids.
![]()
Question 10.
Water behaves as an amphoteric substance in the Bronsted sense. How do you explain?
Answer:
Water can donate a proton and also can accept a proton. So it can act as both acid and base in bronsted sense. A substance which can act both as an acid and a base is called amphoteric substance. Hence, we can say that water is an amphoteric oxide.
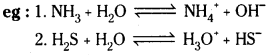
Short Answer Questions
Question 1.
The boiling points of NH3 H2O and HF are higher than those of the, hydrides of the subsequent members of the group. Give your reasons.
Answer:
Hydrogen is covalently bonded with more electronegative atoms such as F, O and N. The weak electrostatic attractive force is called hydrogen bond.
Because of the association of molecules through hydrogen bonds these hydrides have high boiling points. This is because some extra energy is required to break the hydrogen bond.
The electronegativities of the higher members of N, O, F groups are less. So their hydrides are less polar and cannot form hydrogen bonds. Hence the boiling points of the hydrides of the subsequent members of the N, O, F groups have low boiling points.
Question 2.
Discuss the position of hydrogen in the periodic table on the basis of its electronic configuration.
Answer:
Basing on its electronic configuration (1s¹), hydrogen has to be placed in the 1st period in group I along with alkali metals. Hydrogen resembles the alkali metals in its ability to form hydrated uni + ve ion (H+aq).
Hydrogen can also form uni -ve ion (H–). Hence, it can be clubbed with VILA group elements.
However, the position of hydrogen in the periodic table is not satisfactory. It is a matter of choice of the individual to place it in group I along with alkali metals or with group VII along with halogens. Some prefer to place hydrogen separately at the top of the periodic table.
Question 3.
How is the electronic configuration of hydrogen suitable for its chemical reactions?
Answer:
The electronic configuration of hydrogen is 1s¹. It can participate in reactions (i) by losing one electron, (ii) by gaining one electron and (iii) by sharing its electrons.
eg : 1. By losing electron :
In the reaction with fluorine it gives electron but H+ ion cannot exist independently but in water it exist by combining with water.
HF + H2O → H3O+ + F–
2. By gaining electron :
With highly electropositive metals it form hydrides by gaining electron.
Na+ + H– → NaH
3. By sharing electrons. It forms sev¬eral compounds by sharing electrons.
H : Cl
Question 4.
What happens when dihydrogen reacts with a) Chlorine and b) Sodium metal? Explain.
Answer:
a) When chlorine react with hydrogen hydrogen chloride is formed.
H2 + Cl2 → 2HCl
In HCl, hydrogen shares its electron with chlorine. Hydrogen reduces chlorine to HCl.
b) When sodium reacts with hydrogen, sodium hydride is formed.
2Na + H2 → 2NaH
In this reaction, sodium loses electron while hydrogen gains electron forming Na+ and H– ions. Here sodium is oxidised while hydrogen is reduced.
Question 5.
Write a note on heavy water.
Answer:
Heavy water is deuterium oxide. Its formula is D2O. It contains heavier deuterium atoms in the place of normal, hydrogen atoms of water molecule. It is prepared by the prolonged repeated electrolysis of \(\frac{M}{2}\) NaOH solution. The electrolytic cell consists of a steel tank which acts as cathode. A perforated nickel sheet acts as anode and 0.5M NaOH solution acts as electrolyte. The electrolysis is carried in 7 stages. At the end of seventh stage 99% heavy water is obtained.
Properties:
1) It reacts with CaC2 and liberates heavy acetylene.
CaC2 + 2D2O → C2D2 + Ca (OD)2
2) It reacts with S03 and produce heavy sulphuric acid.
SO3 + D2O → D2SO4
Hydrolysis reactions of salts with heavy water are called deuterolysis reactions.
Ex : AlCl3 + 3D2O → Al(OD)3 + 3DCl
Uses :
It is extensively used in nuclear reactors as a moderator and in exchange reactions for the study of reaction mechanism.
![]()
Question 6.
Name the isotopes of hydrogen. What is the ratio of the masses of these isotopes?
Answer:
Hydrogen 1¹H
Deuterium 1²H or 1²D
Tritium : 1³H or 1³T
The masses of these isotopes are H = 1.008,
D = 2.014 and T = 3.016.
Question 7.
What is water – gas shift reaction? How can the production of dihydrogen be increased by this reaction?
Answer:
Generally water gas produced by passing steam over red hot coke contain less percentage of H2.
C + H2O → CO + H2
The amount of H2 in water gas can be increased by reacting CO of syngas with steam in the presence of Iron chromate catalyst.
CO (g) + H2O (g) → CO2 + H2. This is called water gas shift reaction.
The CO2 formed is removed by scrubbing with sodium arsenite solution.
Question 8.
Complete and balance the following reactions :
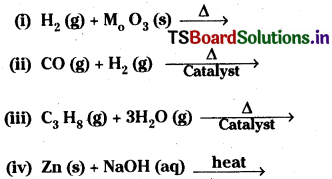
Answer:
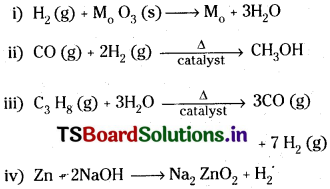
Question 9.
What is the nature of the hydrides formed by elements of 13 group?
Answer:
Group 13 elements form covalent hydrides. These are mainly electron deficient hydrides. They act as Lewis acids.
Boron form large number of hydrides called boranes. Aluminium form a single polymeric hydride called allane. Gallium forms a dimerichydride and indium forms a polymeric hydride which are not stable. Thallium does not from any hydride.
Question 10.
Discuss the principle and the method of softening of hard water by synthetic, ion-exchange resins.
Answer:
The water which is free from all the dissolved mineral salts is called deionised water.
Deionised water can be prepared in two steps.
1) Removal of cations :
Hard water is passed through a tank containing cation exchange resin. Then the Ca+2 and Mg+2 ions present in the water are replaced by H+ ions from the resin.
2R COOH + Ca+2 → [R (COO)]2 Ca + 2H+
2) Removal of anions:
Now the water coming from the cation tank is passed through a tank containing anion exchange resin. This resin absorbs anions like Cl–, SO-24 etc. from the hard water and OH– ions are released.
RNH+3 OH– + Cl– → RNH+3 Cl– + OH–
2RNH+3 OH– + SO-24 → (R – NH+3)2 SO-24 + 2OH–
The H+ and OH– ions unite to form Deionised water.
After some time the cation resin and the anion resin lose their capacities to remove the cations and anions from the hard water. Then the resins are said to be exhausted. Then they are to be regenerated. The cation resin is regenerated by passing a solution of H2SO4. Anion resin is regenerated by passing a moderately concentrated solution of NaOH.
![]()
Question 11.
Write a few lines on the utility of hydrogen as a fule. [Mar. ;13]
Answer:
Hydrogen releases large quantity of heat energy on combustion. Hence it is widely used as industrial fuel. Moreover, the pollutants released in the combustion of hydrogen will be less. Atomic hydrogen and oxy-hydrogen torches are used for welding and cutting metals. Hydrogen is also used as rocket fuel. It is also used in fuel cells for generating electric energy. On combination with carbon monoxide (water gas) it is used as industrial fuel.
Question 12.
A 1% solution of H2O2 is provided to you. What steps do you take to prepare pure H2O2 from it?
Answer:
The concentration of H2O2 involves three stages.
Stage (1) :
The dilute solution of H2O2 is carefully evaporated on a water bath under reduced pressure. Then 30% H2O2 solution is obtained.
Stage (2):
The 30% H2O2 solution is heated in a distillation flask under reduced pressure. Then 90% H2O2 is obtained.
Stage (3) :
The 90% H2O2 solution is subjected to crystallisation using solid CO2 and ether. Then 100% H2O2 separates out.
Question 13.
Mention any three uses of H2O2 in modern times.
Answer:
- Used as antiseptic in medicine and surgery.
- Used to bleach silk, wool, ivory, hair etc.
- Used to restore the colour of old and spoiled lead paintings.
- Used as an oxidising agent in the labo¬ratory.
Long Answer Questions
Question 1.
White an essay on the commercial preparation of dihydrogen. Give balanced equations.
Answer:
Commercially hydrogen is prepared by the following methods.
1) Electrolysis method :
Electrolysis of . acidified or alkaline water gives hydrogen.
![]()
2) It is obtained as a byproduct during the manufacture of sodium hydroxide.
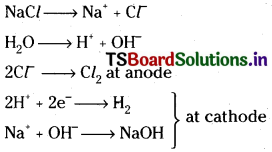
3) By passing a mixture of steam and hydrocarbons over a catalyst gives a mixture of CO and H2.
CH4 + H2O → CO + 3H2
CnH2n+2 + nH2 → nCO + (2n + 1) H2
4) When steam is passed over red hot coke water gas is formed.
C + H2O → CO + H2
The hydrogen present in water gas obtained from hydrocarbons and steam or coke and steam is separated by water gas shift reaction. The water gas is mixed with steam and passed over iron chromate catalyst. Then CO converts into CO2 which is removed by scrubbing with sodium arsenate.
CO + H2O → CO2 + H2
Question 2.
Illustrate the chemistry of dihydrogen by its reaction with
i) N2
ii) Metal ions and metal oxides and
iii) Organic compounds
How is dihydrogen used in the manufac-ture of chemicals?
Answer:
i) Reaction with N2:
Hydrogen react with nitrogen in the presence of iron powder as catalyst forming ammonia.
![]()
This reaction is used for the manufacture of ammonia by Haler’s process.
ii) Reaction with metal ions, metal oxides:
Hydrogen is a good reducing agent and reduces several metal oxides and metal ions to their corresponding metals.
Pd2+ + H2 → Pd + 2H+
CuO + H2 → CU + H2O
iii) Organic compounds :
Hydrogen will be added to the double bonds and triple bonds present in the unsaturated organic compounds in the presence of catalyst.
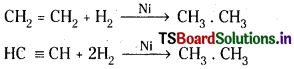
A mixture of H2 and CO is added to 1 – alk- ene to give aldehydes which are further reduced to alcohols. This reaction is known as hydroformylation.
R CH = CH2 + H2 + CO → R CH2 – CH2 CHO
R CH2 CH2 CHO + H2 → R CH2 CH2 CH2OH
In the manufacture of chemicals:
a) HCl is manufactured by burning H2 in Cl2.
H2 + Cl2 → 2HCl
b) Ammonia is manufactured by Haber’s process.
![]()
c) Methyl alcohol is manufactured by passing a mixture of CO and H2 over catalyst.
![]()
Question 3.
Explain, with suitable examples, the following ; [AP Mar. ’19; (AP ’16; IPE ’14)]
i) electron-deficient
ii) electron-precise and
iii) electron-rich hydrides
Answer:
Dihydrogen forms molecular compounds with most of the p – block elements. These are three types.
i) Electron-deficient hydrides:
These are formed by elements of 13 group (Boron family). In these hydrides the electrons available are not sufficient for making the bonds. They do not have octet around the central atom. So they act as Lewis acids. Eg Diborane B2H6.
For writing the Lewis structure the number of available electrons are 12 but required are 14.
ii) Electron precise hydrides :
The hydrides whose central atom is having octet and all the electron pairs around the central are bond pairs are called electron precise hydrides. Eg: CH4, SiH4, CeH4, SnH4. Electron precise hydrides have required number of electrons for writing the Lewis diagrams.
iii) Electron rich hydrides :
The hydrides whose central atom is having octet and among the electron pairs around the central atom if some are bond pairs and some are lone pairs are called electron rich molecule. Eg NH3, H2O. These contain excess electrons which are present as lone pairs.
![]()
Question 4.
Write in brief on [AP Mar. ’19]
i) ionic hydrides
ii) interstitial hydrides.
Answer:
i) Ionic hydrides :
These are formed by the highly electropositive s – block metals. In s- block metal hydrides LiH, BeH2 and MgH2 have some covalent character. The ionic hydrides are crystalline. They are non-volatile compounds. They are non-conducting in solid state. But in molten condition, if electrolysed they give metal at cathode and hydrogen at anode.
![]()
This reaction confirms that ionic hydrides contain H– ion.
Ionic hydrides liberate hydrogen forming alkaline solution when dissolved in water.
NaH + H2O → NaOH + H2
So ionic hydrides are used as a source of hydrogen.
ii) Interstitial hydrides:
These are formed by many of d – and f – block elements. But metals of 7, 8 and 9 groups do not form hydride. This part of the periodic table is called hydride gap. These are non-stoichiometric compounds. Earlier it was thought the hydrogen atoms occupy the interstitial voids of metal crystals. So they are as interstitial compounds. This is correct only in the case of Ni, Pd, Ce and Ac. In most of other cases the crystal structure of metals is changing during the formation of these hydrides.
Some metals like Pd and Pt absorb large amounts of hydrogen. This property is known as occlusion. This property is also used as a storage of H2 and purification of H2. Also used in catalytic reduction / hydrogenation reactions.
Question 5.
Explain any four of the chemical properties of water.
Answer:
1. Amphoteric nature:
Water can act both as acid and base. In the Bronsted sense it acts as an acid with NH3 and a base with H2S.
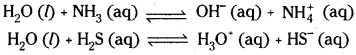
2. Reaction with Metals:
Water react with metals liberating hydrogen. Here water is reduced to hydrogen and metals is oxidised.
2Na + 2H2O → 2NaOH + H2
3. Hydrolysis reaction :
Several ionic and covalent compounds hydrolyse in water.
P4O10 + 6H2O → 4H3PO4
SiCl4 + 2H2O → SiO2 + 4HCl
4. Hydrates formation:
When several salts crystalysed as hydrated salts
Eg : i) [Cr (H2O)6] Cl3 – Coordinated water
ii) BaCl2.2H2O – Lattice water
iii) [Cu (H2O)4] SO4 . H2O. The H2O molecule outside the coordination sphere is hydrogen bonded water.
Question 6.
Explain the terms hard water and soft water. Write a note on the [Mar. ’18 (AP)]
i) ion-exchange method and
ii) Calgon method for the removal of hardness of water. [AP ’16; TS ’15 TS Mar. ’19]
Answer:
Water which gives ready and permanent lather with soap is called soft water.
Water which do not give ready and permanent lather with soap is called hard water.
Hardness of water is due to the presence of soluble chloride, sulphate and bicarbonate compounds of magnesium and calcium such as MgCl2, MgSO4, Mg(HCO3)2, CaCl2, CaSO4, Ca(HCO3)2.
1) Ion exchange method :
The water which is free from all the dissolved mineral salts is called deionised water.
Deionised water can be prepared in two steps.
1) Removal of cations :
Hard water is passed through a tank containing cation exchange resin. Then the Ca+2 and Mg+2 ions present in the water are replaced by H+ ions from the resin.
2R COOH + Ca+2 → [R (COO)]2 Ca + 2H+
2) Removal of anions:
Now the water coming from the cation tank is passed through a tank containing anion exchange resin. This resin absorbs anions like Cl–, SO-224 etc. from the hard water and OH– ions are released.
RNH33 OH– + Cl– → RNH+3 Cl– + OH–
2RNH+3 OH– + SO-224 → (R – NH+3)2 SO-224 + 2OH–
The H+ and OH– ions unite to form Deionised water.
After sometime the cation resin and the anion resin lose their capacities to remove the cations and anions from the hard water. Then the resins are said to be exhausted. Then they are to be regenerated. The cation resin is regenerated by passing a solution of H2SO4. Anion resin is regenerated by passing a moderately concentrated solution of NaOH.
2) Calgon method :
Sodium hexametaphos- phate is commercially called as calgon.
When calgon is added to hard water it reacts with calcium and magnesium ions forming complex anions.
Na2 [Na4 (PO3)6] + 2Mg2+ → Na2 [Mg2 (PO3)6] + 4Na+
Na2 [Na4 (PO3)6] + 2Ca2+ → Na2 [Ca2 (PO3)6] + 4Na+
Due to the formation of the complex the Mg2+ and Ca2+ become inactive and cannot react with soap. So the water can give good lather. This method is used only for laundry process.
Question 7.
Write the chemical reaction to justify that hydrogen peroxide can function as an oxidizing as well as reducing agent. [TS Mar. ’18, (TS ’16; AP ’15]
Answer:
Hydrogen peroxide can act as both oxidising and reducing agent in both acid and basic medium.
Oxidation properties:
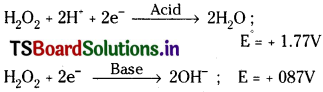
Eg : H2O2 oxidises PbS to PbSO4 in acid medium
PbS + 4H2O2 → PbSO4 + 4H2O.
H2O2 oxidises formaldehyde to formic acid in basic medium.
2HCHO + H2O2 → 2HCOOH + H2
Reduction properties:
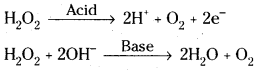
Eg : H2O2 reduces acidified potassium per-manganate in acid medium.
2KMnO4 + 3H2SO4 + 5H2O2 → K2SO4 + 2MnSO4 + 8H2O + 5O2
H2O2 reduces potassium ferrycyanide in basic medium.
2K3 [Fe (CN)6] + 2KOH + H2O2 → 2K4[Fe (CN)6] + 2H2O + O2
Question 8.
Complete and balance the following chemical reactions:
i) PhS (s) + H2O2(aq) →
ii) MnO–4 (aq) + H2O2 (aq) →
iii) CaO (s) + H2O (g) →
iv) Ca3 N2 (s) + H2O (l) →
Classify the above into (a) hydrolysis (b) redox and (c) hydration reactions.
Answer:
i) PbS + 4H2O2(aq) → PbSO4 + 4H2O
This reaction is redox reaction. Pbs is oxidised, H2O2 is reduced.
ii) 2MnO–4 (aq) + 6H+ + 5H2O2 (aq) → 2Mn2+ + 8H2O + 5O2
This reaction is redox reaction. Here MnO–4 is reduced while H202 is oxidised.
iii) CaO (s) + H2O (g) → Ca (OH)2
This reaction is hydration.
iv) Ca3 N2 (s) + 6H2O → 3Ca(OH)2 + 2NH3
This is hydrolysis reaction.
![]()
Question 9.
Discuss, with relevant chemical equations, various methods of preparing hydrogen peroxide. Which of these methods is useful to prepare D2 O2?
Answer:
Hydrogen peroxide can be prepared by the following methods.
1) By adding ice cold dilute sulphuric acid to barium peroxide gives hydrogen peroxide.
BaO2.8H2O (s) + H2SO4 (aq) → BaSO4 + H2O2 + 8 H2O
BaSO4 can be removed by filtration. Excess water can be removed by evaporation under reduced pressure.
2) Electrolysis of 50% H2SO4 using platinum anode and lead cathode gives H2O2.
2H2SO4 → 2H+ + 2HSO–4
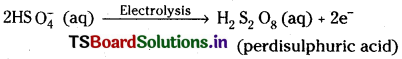
Hydrolysis of perdisulphuric acid gives hydrogen peroxide.
H2S2O8 + 2H2O → 2H2SO4 + H2O2.
3) Industrially it is prepared by the auto oxidation of 2 – alkylanthraquinols.
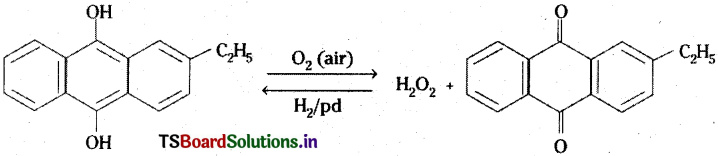
K2S2O8 is used for the preparation of D2O2.
D2O2 can be prepared by dissolving K2S2O6 in heavy water.
K2S2O8 + 2D2O → 2KDSO4 + D2O2
Question 10.
In how many ways can you express the strength of H2O2? Calculate the strength of 15 volume solution of H2O2.in g/l. Express this strength in normality and molarity.
Answer:
i) Strength of H2O2 expressed in volumes:
Eg : 10 vol. H2O2, 20 vol. H2O2 and 100 vol. H2O2
20 vol. H2O2 means 1 ml of this solution liberates 20 ml of O2 gas at STP.
∴ 10 ml of 20 vol liberates 200 ml of oxygen at STP.
10 vol. H2O2 solution means 1 ml of this solution liberates 10 ml of O2 gas at STP.
ii) To express the strength in % (W/V) :
H2O2 decomposes, as shown below
![]()
1 mole of O2 gas is liberated from 2 notes of H2O2.
i.e., 22.4 lit of O2 is given by 2 × 34 g of H2O2.
∴ 10 lit of O2 gas at STP can be given by?
Weight of H2O2 in 1 lit of solution \(\frac{2\times34\times10}{22.4}\) = 30.36 g
30.36g (W/V) refers to the weight of H2O, in 1000 ml of solution.
∴ Strength of H2O2 = 3.036 % (W/V)
(Wt. of H2O2 in 100 ml solution is called its strength.)
iii) Molarity of H2O2 solution = \(\frac{30.36}{34}\) = 0.893 M
iv) Normality of solution is the number of gram equivalents of solute present in 1 lit of solution.
Equivalent weight of H2O2

Strength of 15 vol H2O2 :
10 volume H2O2 is 3% W/V
15 volume H202 is
\(\frac{15\times3}{10}\) = 4.5 gm/100ml
∴ The wt. of H202 in 1 litre = 45 g/L
Normality of 10 vol. H2O2 is 1.786
Normality of 15 vol. H2O2 is
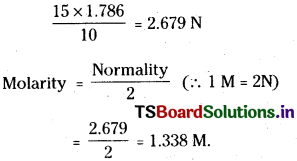
![]()
Question 11.
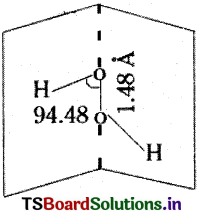
Explain the structure of the Hydrogen peroxide molecule.
Answer:
H2O2 has an open book structure. It is non-linear and non-polar. The two oxygen atoms are considered to be present on the spine of an open book. The two hydrogen atoms can be considered to be present on the two covers. H-O-O bond angle is 94° 48¹. O-O bond length is 1.48 A.