Telangana TSBIE TS Inter 1st Year Chemistry Study Material 7th Lesson Chemical Equilibrium and Acids-Bases Textbook Questions and Answers.
TS Inter 1st Year Chemistry Study Material 7th Lesson Chemical Equilibrium and Acids-Bases
Very Short Answer Type Questions
Question 1.
State law of chemical equilibrium.
Answer:
At a given temperature, the product of concentrations of the products raised to the respective stoichiometric coefficients in the balanced chemical equation divided by the product of the concentrations of the reactants raised to their individual stoichiometric coefficients has a constant value. This is known as the equilibrium law or law of chemical equilibrium.
Question 2.
Can equilibrium be achieved between water and its vapours in an open vessel? Explain.
Answr:
No. Since the vapours escape into atmosphere from the open vessel equilibrium cannot be achieved between water and its vapours.
Question 3.
Why the concentrations of pure liquids and pure solids are ignored from equilibrium constant expressions?
Answer:
In the. heterogeneous equilibria the molar concentrations of pure liquid or a pure solid remain constant. So they are ignored from equilibrium constant expression.
Question 4.
What is homogeneous equilibrium? Write two homogeneous reactions.
Answer:
The equilibrium in which all the substances are present in the same phase is known as ‘ homogeneous equilibrium, eg:
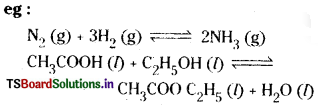
Question 5.
WTiat is heterogeneous equilibrium? Write two heterogeneous reactions. [AP Mar. ’17]
Answer:
The equilibrium in which the substances involved are present in different phases is called heterogeneous equilibrium.
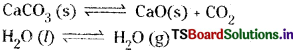
![]()
Questtion 6.
Write reaction quotient, Q, for each of the following reactions.
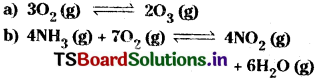
Answer:
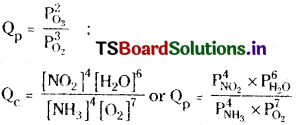
Question 7.
Define equilibrium constant.
Answer:
The equilibrium constant, Kc may be defined as the ratio of product of the equilibrium concentrations of the products to that of the reactants with each concentration term raised to the power equal to the stoichiometric coefficient of the substance in the balanced chemical equation.
Question 8.
The equilibrium constant expression for a gas reaction is Kc = \(=\frac{\left[\mathrm{NH}_3\right]^4\left[\mathrm{O}_2\right]^5}{[\mathrm{NO}]^4\left[\mathrm{H}_2 \mathrm{O}\right]^6}\)
Write the balanced chemical equation corresponding to this expression.
Answer:
![]()
Quesstion 9.
Write the relation between Kp and Kc.
Answer:
Kp = Kc[RT]∆n.
Question 10.
Under what conditions for a reaction Kp and Kc are numerically equal?
Answer:
If the total number of molecules of gaseous products and the total number of molecules of gaseous reactants are equal (∆n – 0) then Kp & Kc are equal
Kp = Kc[RT]∆n
Kp = Kc (∴ ∆n = 0)
![]()
Question 11.
Give two chemical equilibrium reactions for which Kp = Kc
Answer:

Question 12.
Give two chemical equilibrium reactions for which Kp > Kc.
Answer:
In order that Kp < Kc ∆n should be +ve

Question 13.
Give two chemical equilibrium reactions for which Kp < Kc.
Answer:
In order that Kp < Kc, ∆n should be -ve
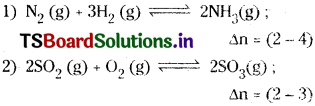
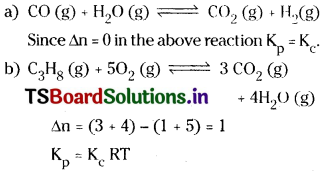
Question 14.
Write the equations for the conversion of Kc to Kp for each of the following reactions.
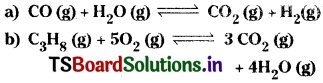
Answer:
Question 15.
What are the factors which influence the chemical equilibrium?
Answer:
Molar concentration, temperature, and pressure influence the equilibrium.
Question 16.
What is the effect of pressure on a gaseous chemical equilibrium? [AP Mar. ’19]
Answer:
When pressure is increased on a gaseous equilibrium the equilibrium shift in the direction of lesser number molecules in the balanced chemical equilibrium reaction.
Question 17.
What is the effect of increase in concentration of reactants of a chemical reaction at equilibrium?
Answer:
If the concentration of one of the substance in equilibrium is increased, the equilibrium shift in the direction so that the concentration of the substance decreases. Thus if concentration of a reactant is increased forward reaction takes place more.
Question 18.
Can catalyst disturb the state of equilibrium?
Answer:
No. A catalyst catalise both forward and backward reactions equally. So equilibrium is not disturbed but is achieved fastly.
Question 19.
On which factor, the equilibrium constant value changes?
Answer:
Temperature. If the forward reaction is exothermic, the backward reaction in the equilibrium is endothermic. Increase temperature favours the endothermic reaction. So the equilibrium constant changes.
Question 20.
The equilibrium constants of a reaction at 27°C and at 127°C are 1.6 × 10-3 and 7.6 × 10-2 respectively. Is the reaction exothermic or endothermic?
Answer:
1.6 × 10-3 < 7.6 × 10-2
As Kc value increases with increase in temperature the reaction is endothermic since endothermic reactions are more favourable at high temperatures.
![]()
Question 21.
What is the effect of temperature on a system at equilibrium?
Answer:
If temperature is increased on the system in the equilibrium if the forward reaction is endothermic, the equilibrium shift in the forward direction. But if the forward reaction is exothermic the equilibrium shift in the backward direction.
Question 22.
For an exothermic reaction, what happens to the equilibrium constant if temperature is raised?
Answer:
In the equilibrium if temperature is raised the exothermic reaction (forward reaction) decreases while the backward reaction (endothermic reaction) takes place more. So Kc decreases.
Question 23.
What kind of equilibrium constant can be calculated from ∆G° Value for a reaction involving only gases?
Answer:
∆G° = -2.303 RT log KC
From the above equation equilibrium constant KC can be calculated.
Question 24.
What is a Bronsted base? Give one example. [TS Mar. 19; (AP ’16)]
Answer:
The substance which can accept a proton is said to be Bronsted base.
Ex : Cl–, HCO–3
Question 25.
What is Lewis acid? Give one example. [Mar. ’18 (TS) (AP ’16, ’15)]
Answer:
Lewis acid is the substance which can accept a pair of electrons and form a coordinate covalent bond.
Ex: BF3, H+
Question 26.
What is meant by ionic product of water? [AP Mar. ’17, ’16]
Answer:
The product of concentration of H+ and OH– ions in aqueous solution (or) in pure water is called ionic product of water.
Question 27.
What is the value of Kw? What are its units?
Answer:
Ionic product of water Kw = 1 × 10-14 Units are mole²/lit²
Question 28.
What is the effect of temperature on ionic product of water?
Answer:
With increase in temperature ionisation of water also increases. So ionic product increases.
Question 29.
![]()
The ionic product of water is 1 × 10-14 at 25°C and 3.0 × 10-14 at 40°C.
Is the above process endothermic or exothermic?
Answer:
With increase in temperature ionic product is increasing. This indicates ionisation of water increases with increase in temperature by absorbing heat energy. So it is an endothermic reaction.
Question 30.
All Bronsted bases are Lewis bases? Explain.
Answer:
A Bronsted base is that which can accept proton. While accepting a proton the Bronsted base donate a lone pair of electrons to proton.
Eg : H3 N : + H+ → H3N : → H
A Lewis base is that which can donate a lone pair of electrons. So all Bronsted bases can also act as Lewis bases.
![]()
Question 31.
All Lewis acids are not Bronsted acids. Why?
Answer:
A Lewis acid is that which accept a lone pair of electrons.
Eg : BF3 can accept a lone pair.
A Bronsted acid is that which donate a proton, eg : HCl. So Lewis acids may not contain proton but can accept lone pair. Hence all Lewis acids are not Bronsted acids.
Question 32.
What is degree of ionisation?
Answer:
The fraction of an ionic substance that undergo ionisation per one mole of that substance in aqueous solution is called degree of ionisation.
Degree of ionisation (α) = number of molecules ionised/number of molecules taken.
Question 33.
What is the measure of strength of an acid and base?
Answer:
The strength of an acid depends on its dissociation constant (Ka) and the strength of a base depends on its dissociation constant (Kb). As the values of Ka or Kb increase, the strengths of acid (or) base increase.
Question 34.
Give two examples of salts whose aqueous solutions are basic.
Answer:
Na2CO3, CH3COONa.
Question 35.
Give two examples of salts whose aqueous salts are acidic.
Answer:
- AlCl3
- CuSO4
Question 36.
What equation is used for calculating the pH of an acid buffer?
Answer:
To calculate the pH of an acidic buffer to equation is pH = pKa + log\(\frac{\mathrm{[Sait]}}{\mathrm{[Acid]}}\).
Question 37.
Phosphoric acid (H3PO4) have three ionization constants Ka1, Ka2 and Ka3. Among these ionization constants which has a lower value? Give reason for it.
Answer:
Ka3 has lower value. This is because it becomes, more difficult to remove a positively charged proton from a negative ion (HPO2-4) due to strong electrostatic attraction.
Question 38.
Ice melts slowly at high altitudes. Explain. Why?
Answer:
Ice has more volume than water. Decrease in pressure decreases the melting point of ice in equilibrium.
![]()
At higher altitudes the atmospheric pressure is less. Hence, ice melts slowly at high altitudes.
Short Answer Questions
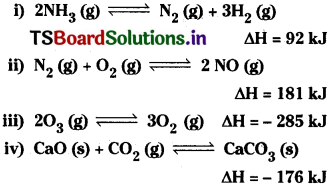
Question 1.
Write expression for the equilibrium constant, Kc, for each of the following reactions.
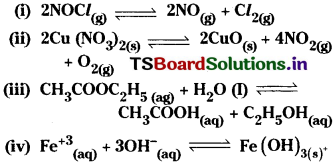
Answer:
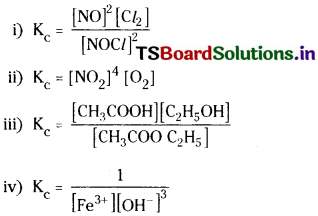
In the above expressions the concentrations of solids and water are taken as unity.
Question 2.
Derive the relation between Kp and Kc for the equilibrium reaction. [TS Mar. ’19; (AP ’15, Mar. ’13)]
![]()
Answer:
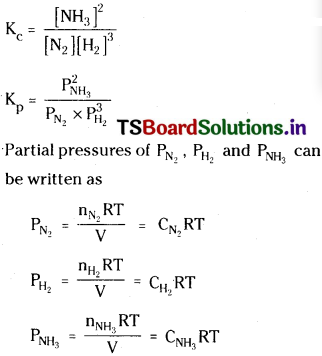
Substituting these values in Kp equation
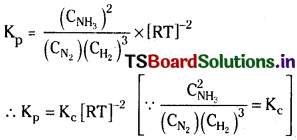
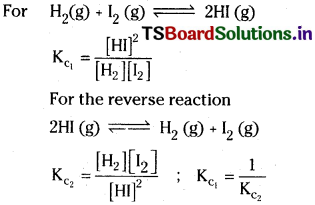
Question 3.
Define equilibrium constant. Write the equilibrium constant expression for the reaction of
![]()
and its reverse reaction. How are the two equilibrium constants related?
Answer:
Equilibrium constant :
The equilibrium constant, Kc may be defined as the ratio of product of the equilibrium concentrations of the products to that of the reactants with each concentration term raised to the power equal to the stoichiometric coefficient of the substance in the balanced chemical equation.
Question 4.
How does the value of equilibrium constant predict the extent of reaction?
Answer:
For a general equation
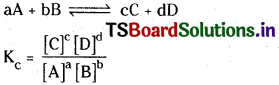
If Kc value is more, the products formed are more. So the forward reaction takes place. If Kc > 1 the forward reaction takes place more and the products formed are more. If the value of Kc < 1 the products formed are less. So the reaction takes place less than 50%.
![]()
Question 5.
State law of chemical equilibrium? What is Kc for the following equilibrium when the equilibrium concentration of each substance is [SO2] = 0.60 M, [O2] = 0.82 M and [SO3] = 1.90 M?
![]()
Answer:
Law of chemical equilibrium :
At a given temperature, the product of concentrations of the reaction products raised to the respective stoichiometric coefficients in the balanced chemical equation divided by the product of concentrations of the reactants raised to their individual stoichiometric coefficient has a constant value.
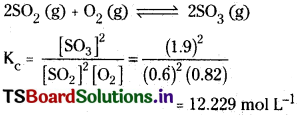
Question 6.
Why sealed soda water bottle on opening shows the evolution of gas with effervescence?
Answer:
In a sealed soda bottle the pressure of CO2 gas will be high. Then the CO2 gas is dissolved in water is more and the CO2 is in equilibrium with water.
![]()
When pressure is decreased the equilibrium shift in the direction where the number of gaseous molecules are more. There is CO2 gaseous molecule on reactants side but no gaseous molecules on products side. So the equilibrium shift in backward direction and the dissolved oxygen will be liberated with effervescence.
Question 7.
Explain the significance of
a) a very large value of K,
b) a very small value of K and
c) a value of K of about 1.0
Answer:
a) When Kc is very large the reaction proceeds nearly to completion. Since Kc is very large when the forward reaction takes place more and backward reaction is very small. So reaction goes to almost completion.
b) If Kc is very small the reaction proceeds rarely when Kc is very small in the equilibrium the forward reaction takes place to a small extent only. So the reaction proceeds to a small extent only.
c) Kc is the ratio of rate constants of forward reaction and rate constant of backward reaction. When the rate constants of both forward backward reactions are equal the value of Kc becomes 1. This indicates that the reaction proceeds to an extent of 50%.
Question 8.
Why is it useful to compare Q with K? What is the situation when
(a) Q = K (b) Q < K (c) Q > K ?
Answer:
The ratio of the product of molar concentrations of the products to the product of molar concentrations of reactants with each concentration term raised to the power equal to the stoichiometric. Coefficient of that species is called reaction quotient.
Comparing the values of Q with K we can predict the direction of reaction.
If Q is greater than K, the net reaction takes place in backward direction whereas if Q is less than K, the net reaction takes place in forward direction. At equilibrium Q = K.
Question 9.
For the reaction
![]()
What will happen in a mixture originally containing [Cl2] = 0.04 mol L–;
[F2] = 0.2 mol L-1 and [Cl F] = 7.3 mol L-1?
Answer:
The reaction quotient
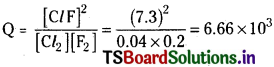
Since the Q value is very high than the Kc value 19.9 the reaction takes place in backward direction.
Question 10.
Predict which of the following reaction will have appreciable concentration of reactants and products:
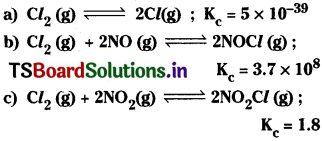
Answer:
a) Since Kc for the reaction
![]()
is very small the reaction proceeding in the forward direction is very very small. So in the equilibrium mainly reactants are present.
b) The Kc value of the reaction
is very high. So the reaction proceeds in the forward direction is more. In the equilibrium, the concentrations of products are very high.
c) The Kc value of the reaction

is nearly 1. So the reaction proceeds in the forward direction a little more than 50%.
Question 11.
How to recognise the conditions under which changes in pressure would effect system in equilibrium.
Answer:
When pressure changes occurs on a chemical reaction in equilibrium, there will be change in the number of gaseous molecules, if there is difference in the total number of gaseous molecules of reactants and products.
If pressure is increased the reaction proceeds in the direction where the number of gaseous molecules are less. Since the volume of a gas is directly proportional to volume with decrease in number of molecules volume also decreases. As the sum of the masses of the substances in the vessel is changed density increases. So by measuring the density of the equilibrium mixture the effect of change in pressure on the equilibrium can be recognised.
![]()
Question 12.
What property of a reaction can be used to predict the effect of a change in temperature on the magnitude of an equilibrium constant?
Answer:
Colour of the reaction mixture can be used to predict the effect of change in temperature on the magnitude of an equilibrium constant.
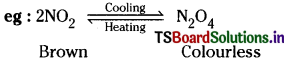
When the temperature decreases dimerisation of NO2 takes place more and thus the value of Kc increases.
Question 13.
Does the number of moles of reaction products increase, decrease, or remains same when each of the following equilibria is subjected to a decrease in pressure by increasing the volume?
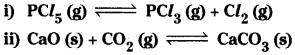
Answer:
i) According to Le Chatelier’s principle if the pressure is decreased on equilibrium, the equilibrium shifts in the direction so that the increased pressure is nullified. Here the number of gaseous molecules (2) of products are more than the number of gaseous molecules (1) of the reactants the reaction proceed in the forward direction. So the number of moles of products increases,
ii) In this reaction gaseous molecule (CO2) is present in the reactant. When pressure is decreased the number of CO2 molecules increase on reactant side. To increase the number of CO2 moles the CaCO3 should decompose. Thus the number of moles of products decrease.
Question 14.
Which of the following reactions will get affected by increasing the pressure? Also mention whether change will cause the reaction to go into forward or backward direction.
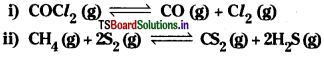
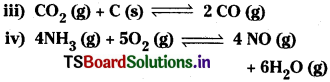
Answer:
According to Le Chatelier’s principle when presssure is increased on equilibrium, the equilibrium shift in the direction where the number of gaseous molecules are less.
![]()
In this reaction the number of gaseous reactant molecules (1) are less than the total number of gaseous molecules (2) of products. So increase pressure shifts the equilibrium in backward direction.
![]()
In this reaction the total number of gaseous reactant molecules (3) are equal to the total number of gaseous product molecules (3). So pressure has no effect on this equilibrium.
![]()
In this reaction the number of gaseous reactant molecules (1) is less than the total number of gaseous product molecules (2). So increase in pressure shifts the equilibrium in the backward direction.
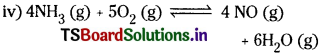
In this reaction the sum of the number of gaseous reactant molecules (g) is less than the sum of the number of the gaseous product molecules (10). So the increase in pressure shifts the equilibrium in the backward direction.
Question 15.
How will an increase in pressure and affect each of the following equilibria? an increase in temperature
Answer:
According to Le Chatelier’s principle increase in pressure shifts the equilibrium in the direction where the number of gaseous molecules are less.
Increase in temperature favours the endothermic reaction while decrease in temperature favours the exothermic reaction.
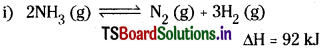
The number of gaseous reactant molecules (2) is less than the sum of the total number of product molecules (4). So increase in pressure shifts the equilibrium in the backward direction.
Forward reaction is endothermic while the backward reaction is exothermic. So increase temperature shifts the equilibrium in the forward direction.
![]()
The sum of the gaseous reactant molecules is equal to the sum of the gaseous product molecules. So pressure has no affect on the equilibrium.
Forward reaction is endothermic while backward reaction is exothermic. So increase in temperature favours the reaction.
![]()
The number of gaseous reactant molecules (2) is less than the number of gaseous product molecules. So increase in pressure favours the formation of O3.
The forward reaction is exothermic. While the backward reaction is endothermic. So increase in temperature favours the backward reaction.
![]()
There is only one gaseous reactant molecule in this reaction. No gaseous product molecules. So increase in the pressure shift the equilibrium in forward direction.
The forward reaction is exothermic while the backward reaction is endothermic. So increase in pressure shifts the equilibrium in the backward direction.
Question 16.
The dissociation of HI is independent of pressure, while the dissociation of PCl5 depends upon the pressure applied explain.
Answer:
According to Le Chatelier’s principle increase in pressure shifts the equilibrium in the direction where the number of gaseous molecules are less and decrease in pressure shift the equilibrium in the direction where the number of gaseous molecules are more.
![]()
In this reaction the sum of the number of gaseous reactant molecules and the total number of gaseous product molecules is equal. So pressure has no effect on this equilibrium.
Dissociation of PCl5:
![]()
In this reaction the no. of gaseous reactant molecules (1) is less than the sum of the gaseous product molecules (2). So increase in pressure shifts the equilibrium in the backward direction. So increase in pressure decreases the dissociation of PCl5
![]()
Question 17.
Explain the terms:
(i) Electrolyte
(ii) Non-electrolyte
(iii) Strong and weak electrolytes
(iv) Ionic equilibrium
Answer:
i) Electrolyte:
The substances which conduct electricity in their aqueous solutions are called electrolytes.
ii) Non-electrolyte:
The substances which do not conduct electricity in their aqueous solutions are called non-electrolytes.
iii) Strong and weak electrolytes :
The electrolytes which are almost completely ionised in solutions are called strong electrolytes. The electrolytes weakly ionise in their solutions are called weak electrolytes.
iv) Ionic equilibrium:
Weak electrolytes when dissolved in water ionise partially and there exists an equilibrium between the ions formed due to ionisation and the undissociated molecules. This is known as ionic equilibrium.
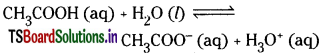
Question 18.
Explain the terms:
i) extent of ionization and on what factors it depends.
ii) dissociation
iii) ionization
Answer:
i) Weak electrolytes are ionised only partially. The ions produced as a result of dissociation of weak electrolytes are present in dynamic equilibrium with the undissociated molecules.
The fraction of total number of molecules of the electrolyte dissolved, that ionises at equilibrium is called degree of ionisation or degree of dissociation. The percent of ionised molecules is called extent of ionisation.
The factors that infuence the extent of ionisation are
a) Concentration :
With decrease in concentration or increase in dilution increases the ionisation.
b) Temperature:
Ionisation increases with increase in temperature.
ii) Dissociation:
Certain compounds on heating decomposes.
Eg : CaCO3 → CaO + CO3
This is known as dissociation or decomposition.
iii) Ionisation:
Certain compounds dissociate into two types of particles when dissolved in water. One type of particles carry positive charge and they are called positive ions. The second type of particles carry an equal amount of negative charge and they are called negative ions. This process is called ionisation.
Question 19.
Explain the Arrhenius concept of acids and bases.
Answer:
According to Arrhenius concept,
An acid is a substance which can furnish hydrogen ions in its aqueous solution.
A base is a substance which can furnish hydroxyl ions in its aqueous solution. Substances such as HNO3, HCl, CH3COOH are acids.
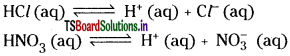
The substances such as NaOH, NH4OH are bases.

Acids such as HCl and HNO3 which are almost completely ionised in aqueous solutions are strong acids whereas acids such as CHgCOOH which are weakly ionised are called weak acids.
Similarly, bases which are almost completely ionised in aqueous solutions are termed as strong bases whereas bases NH4OH are only slightly ionised and are called weak bases.
According to Arrhenius theory, neutralisation of acids and bases is basically a reaction between H+ and OH– ions solutions.
![]()
Question 20.
What is a conjugate acid-base pair? Illustrate with examples.
Answer:
An acid after losing a proton becomes a base whereas a base after accepting the proton becomes an acid.

A base formed by the loss of a proton by an acid is called conjugate base of the acid.
An acid formed by gain of a proton by the base is called conjugate acid of the acid.
Acid-base pairs such as H2O/OH– and NH–4 /NH3 which are formed by loss or gain of a proton are called conjugate acid-base pair.
An acid-base pair which differ by one proton is called conjugate acid base pair.
In the above example H2O / OH– and NH–4 /NH3 are conjugate acid-base pairs.
A strong acid would have large tendency to donate proton. Thus conjugate base of a strong acid would be a weak base. Similarly, conjugate base of a weak acid would be a strong base.
Question 21.
Acetic acid is a weak acid. List, in order of descending concentration, all of the ionic and molecular species present in 1 M aqueous solution of acetic acid.
Answer:
Acetic acid ionises in water as
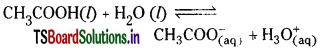
Since acetic acid is weak electrolyte, it ionises to a lesser extent. So the concentration of the ionic and molecular species present in 1M aqueous solution of acetic acid will be in the order
[H2O] > [CH3COOH] > [H3O+] = [CH3COO–] > OH–
Question 22.
Show by suitable equations that each of the following species can act as a Bronsted acid.
a) H3O+ b) HCl c) NH3
Answer:
a) H3O+ + OH– → 2H2O
H3O+ is donating proton to OH” ion. So it is Bronsted acid.
b) HCl + H2O → H3O+ + Cl–
Since HCl is donating proton to H2O molecule, it is a Bronsted base.
c) NH3 + NH3 → NH4 + NH2
Here NH3 is giving H+ ion to another NH3 molecule. So it is Bronsted base.
Question 23.
Show by suitable equations that each of the following species can act as a Bronsted base.
a) H2O
b) OH–
c) C2H5OH
d) HPO-24
Answer:
![]()
In this reaction one water molecule is accepting the proton from the other water moelcule. So the water molecule accepting the proton is a Bronsted base.
b) HCl + OH– → H2O + Cl–
Here OH– is accepting the proton from HCl. So it is Bronsted base.

In Protonation of ethyl alcohol, it is acting as Bronsted base.
d) HPO2-4 + HCl → H2PO2-4 +Cl–
Since HPO2-4 accept the proton from HCl it is Bronsted base.
![]()
Question 24.
The species H2O, HCO–3, HSO–4 and NH3 can act both as Bronsted acids and bases. Give the corresponding conjugate acid and base for each of them. [Mar. ’18 (AP)]
Answer:
- H3O+/H2O and H2O/OH–
- H2SO4/HSO4 and HSO–4/SO2-4
Question 25.
Write equation that shows H2PO–4 acting both as an acid and as a base.
Answer:
H2PO–4 + H2O → HPO2-4 + H3O+
Here H2P–4 is giving proton to water so it is an acid.
H2PO–4 + HCl → H3PO4 + Cl–
Here H2PO–4 is taking proton so it is a base.
Question 26.
Write the conjugate acid and conjugate base of each of the following:
a) OH– b) H2O C) HCO–3 d) H2O2
Answer:
a) Conjugate acid of OH- is H2O
Conjugate base of OH– is O2-
b) Conjugate acid of H2O is H3O+
Conjugate base of H2O is OH–
c) Conjugate acid of HCO–3 is H2CO3
Conjugate base of HCO–3 is CO2-3
d) Conjugate acid of H2O2 is H3O+2
Conjugate base of H2O2 is HO–2
Question 27.
Identify and label the Bronsted acid and its conjugate base, the Bronsted base and its conjugate acid in each of the following equations.
a) H2SO4 + Cl– → HCl + HSO–4
b) H2S + NH–2 → HS– + NH3
c) CN– + H2O → HCN + OH–
d) O2- + H2O → 2OH–
Answer:
a) H2SO4 + Cl– → HCl + HSO–4
H2SO4 is Bronsted acid and its conjugate base is HSO–4. HCl is Bronsted acid and its conjugate base is Cl–
b) H2S + NH–2 → HS– + NH3
H2S is Bronsted acid and its conjugate base HS–
NH3 is Bronsted acid and its conjugate base is NH–2
c) CN– + H2O → HCN + OH–
Here H2O is donating proton. So it is Bronsted acid CN– is accepting proton. So it is Bronsted base.
d) O2- + H2O → OH– + OH–
H2O is donating proton so it is Bronsted acid.
O2- is accepting proton so it is Bronsted base.
Question 28.
Classify the species AlCl3, NH3, Mg+2 and H2O into Lewis acids and Lewis bases and justify your answer?
Lewis acids are those which accept lone pair of electrons and Lewis bases are those which donate lone pair of electrons.
AlCl3 can accept lone pair. So it is Lewis acid.
NH3 can donate a lone pair. So it is Lewis base.
Cations can accept lone pair of electrons. So Mg2+ is Lewis acid.
H2O can donate a lone pair. So it is a Lewis base.
Question 29.
What are the strengths of conjugate bases of a strong acid and a weak acid?
Answer:
An acid which gives a proton easily is a strong acid. A conjugate base which cannot hold the proton strongly is a weak base. So the conjugate base of strong acid is weak base.
A strong base is that which can hold the proton strongly. So the conjugate base of a weak acid is stronger base.
Question 30.
What are the strengths of conjugate acids of a strong base and weak base?
Answer:
A strong base is that which holds the proton strongly. So its conjugate acid is a weak acid.
A weak base is that which cannot hold the proton strongly. So its conjugate is a strong acid.
Question 31.
Define ionic product of water. What is its value at room temperature?
Answer:
The product of molar concentration of H+ ions and OH– ions at a given temperature is called ionic product of water. It is denoted by Kw.
Kw = [H+] [OH–]
at 25°C the Kw of pure water = 1 × 10-14 mol²/lit².
Question 32.
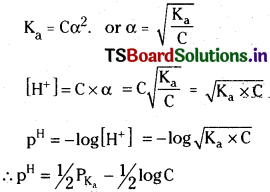
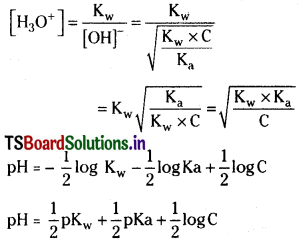
Define pH. pH cannot be calculated directly from the molar concentration of a weak acid or weak base. Why? Derive an equation for the pH of a weak acid.
Answer:
The pH of a solution is defined as the negative logarithm to base 10 of hydrogen ion concentration (or) pH = -log10[H+],
Strong acids and strong bases ionise completely in their aqueous solutions. So the molar concentrations of strong acids or strong bases are equal to the molar concentrations of H+ and OH– ions produced by them.
Weak acids and weak bases do not ionise completely. So the molar concentrations of H+ ions or OH– ions produced by them are not equal to the molar concentrations of weak acids or bases from which they are produced. Hence pH of the aqueous solutions of weak acid and weak bases cannot be calculated from their molar concentrations.
pH of a weak acid :
Ionisation of weak acid
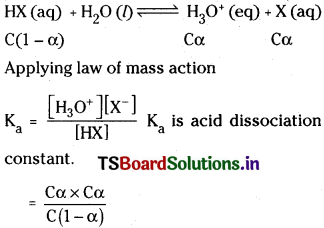
Since the weak acid a is very small as compared to 1, a in the denominator can be neglected. Then
Question 33.
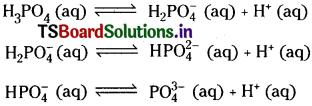
Write equations to show the step wise ionization of the polyprotic acids H2SO4 and H3PO4.
Answer:
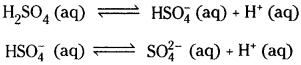
Question 34.
Explain how acid strength changes among Q) the hydrides of the group elements and (ii) the hydrides in the same row of the periodic table.
Answer:
In the hydrides of the elements in a group of the periodic table as we move down the group atomic size increases. So bond length increases. With increase in bond length bond strength decreases. So the ionisation becomes easier.

In a period of the Periodic table as we move from left to right H – A bond polarity increases. So the ionic character of the H – A bond increases, thus ionisation becomes easier. So the strength increases.
![]()
Electronegativity of A increases.
Question 35.
Justify the statement that water behaves like an acid and also like base on the basis of protonic concept.
Answer:
- HCl(aq) + H2O(aq) → H3O+(aq) + Cl–(aq)
- H2O (l) + CO2-3(aq) → HCO–3(aq) + OH–(aq)
In the reaction (1) H2O is behaving as a base whereas in equation (2) it is behaving as an acid. Such substances which can act as acids as well as bases are called amphoteric substances.
![]()
Question 36.
What is common ion effect? Illustrate.
Answer:
The phenomenon of suppression of solubility of an electrolyte in water by the addition of another electrolyte which has a common ion with the original electrolyte is called common ion effect.
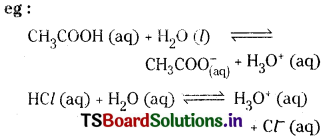
Acetic acid is a weak electrolyte. By adding HCl a strong electrolyte which ionises completely provides more H3O+ concentration. According to Le Chatelier’s principle due to increase in H3O+ concentration, the equilibrium of the weak electrolyte (CH3COOH) shift in the backward reaction thus decreasing the ionisation of acetic acid.
Question 37.
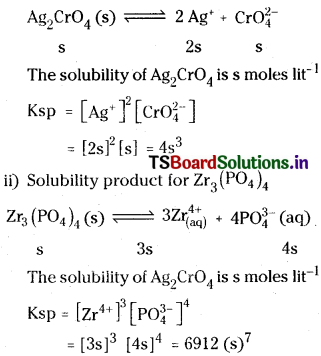
Define solubility product. Write solubility product expressions for the following:
i) Ag2Cr2O7
ii) Zr3(PO4)4
Answer:
Solubility product :
The product of the concentrations of the cation and anion in a saturated solution of a salt at room temperature, is called solubility product.
i) Solubility product for Ag2CrO4
Question 38.
Give the classification of salts. What type of salts undergo hydrolysis?
Answer:
Salts are classified into 4 types
- Salt of strong acid and strong base
- Salt of strong acid and weak base
- Salt of weak acid and strong base
- Salt of weak acid and weak base.
Salts of strong acid and weak base, strong base and weak acid, weak acid and weak base hydrolyse in water.
Question 39.
What must be true of the value of ∆ G° for a reaction if
a) K > 1 b) K = 1 c) K < 1 ?
Answer:
a) If K > 1, ∆G° value will become negative. So the reaction is spontaneous or the reaction proceeds in the forward direction more. The products are present predominantly.
b) If K = 1, ∆G° value will become zero. So the reaction proceed neither in the forward direction nor in the backward directiort. The reaction is in equilibrium.
c) If K < 1, ∆G° value will become positive. This indicates that the reaction is non spontaneous or the reaction proceed in the forward direction to a small extent only. The products are present in minute amounts only.
Question 40.
Aqueous solution of NH4Cl is acidic. Explain.
Answer:
Ammonium chloride when dissolved in water react with water forming weak base ammonium hydroxide and strong acid HCl.
![]()
HCl is a strong acid ionising completely but NH4OH being weak base do not ionise completely. So there remains some excess H+ ions in the solution. Due to this reason the aqueous solution of ammonium chloride is acidic.
Question 41.
Aqueous solution of CH3COONa is basic. Explain. [TS ’16]
Answer:
Sodium acetate when dissolved in water ionises completely and the ions formed react with water forming weak acid and strong base.
![]()
Acetic acid being weak acid ionise partially but sodium hydroxide is strong electrolyte and ionises completely. So there remains some excess OH– in solution. Hence the solution of sodium acetate is basic in nature.
Question 42.
Give reason that acetic acid is less acidic in sodium acetate solution than in sodium chloride solution.
Answer:
The ionic equilibrium reactions of acetic acid, sodium acetate and sodium chloride are as follows
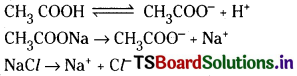
Acetic acid is weak electrolyte and there exists equilibrium. Sodium acetate being strong electrolyte ionises completely furnishing acetate ions. Due to the common ion effect of acetate ions the ionisation of acetic acid decreases. So acetic acid behave as weak acid in the presence of sodium acetate. Sodium chloride do not give any ions common in the ionisation equilibrium of acetic acid. So NaCl has no effect on the ionisation of acetic acid, thereby no effect on acid strength. Hence acetic acid is more acidic in sodium chloride.
Question 43.
AgCl is less soluble in AgNO3 solution than in pure water. Explain.
Answer:
The ionisation equilibrium of AgCl can be written as
![]()
In the saturated solution ionic product [Ag+] [Cl–] is equal to the solubility product Ksp of AgCl.
Similarly the ionisation equilibrium of AgN03 can be written as
![]()
By the addition of AgNO3 to AgCl the number of Ag+ increases and the ionic product [Ag+] [Cl–] exceeds the solubility product of AgCl. So precipitation occurs causing the decrease in solubility of AgCl.
Question 44.
Predict whether the following reaction will proceed from left to right to any measurable extent:
CH3COOH (aq) + Cl– (aq) →
Answer:
CH3COOH (aq) + Cl– (aq) → CHgCOO- + HCl
CH3COOH is a weak acid than HCl. This indicates the conjugate base CH3COO– of CH3COOH is strong and holds the proton strongly. HCl is a strong acid. So its conjugate base Cl– ion is weak base. So the reaction cannot proceed from left to right to a measurable extent.
Question 45.
Aqueous solution of H2S contains H2S, HS–, S2-, H3O+, OH– and H2O in varying concentrations. Which of these species can act only as a base? Which can act only as an acid? Which can act both as an acid and as a base?
Answer:
a) S2- can act only as a base. It cannot act as acid because it cannot give H+ ions.
b) H2S and H3O+ can act only as acids.
c) HS–, OH– and H2O can act both acids and bases as they can give H+ ions and can accept H+ ions.
Long Answer Questions
Question 1.
What are equilibrium processes? Explain equilibrium in Physical and Chemical processes with examples.
Answer:
In a reversible process, a state will be attained such that therate of forward reaction is equal to the backward rate of reaction. Such a state is known as equilibrium state.
Equilibrium processes are of two types. They are
1. Physical equilibrium process:
In such a process, equilibrium occurs in a phase transformation process.
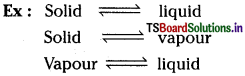
2. Chemical equilibrium process:
If there exists an equilibrium in between reactants and products, such reactions are said to be chemical equilibrium process.
The value of equilibrium constant K changes with change in temperature.
![]()
Question 2.
What is meant by dynamic equilibrium? Explain with suitable examples.
Answer:
In a reversible process, a state will be attained such that the rate of forward reaction is equal to the backward rate of reaction. Such a state is known as equilibrium state.
At equilibrium we find no change in the concentration of either reactants (or) products then we may feel that the reaction has stopped. But actually the reaction will be going on. Such a state of reaction is called dynamic equilibrium.
Equilibrium processes are of two types. They are:
1. Physical equilibrium process: In such a process, equilibrium occurs in a phase transformation process.
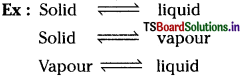
2. Chemical equilibrium process:
If there exists an equilibrium in between reactants and products, such reactions are said to be chemical equilibrium process.
The forward and backward reactions of a reversible reaction continuously takes place with equal rates simultaneously at the equilibrium stage also. Hence the equilibrium is called dynamic equilibrium. At this stage the reaction appears to be stopped.
All chemical equilibrium processes are dynamic equilibriums in nature.
Question 3.
Give the general characteristics of equilibria involving physical processes.
Answer:
General characteristics of equilibria involving physical processes are
1) In the case of liquid ![]() gas equilibrium, the vapour pressure of the liquid is constant at equilibrium at a particular temperature.
gas equilibrium, the vapour pressure of the liquid is constant at equilibrium at a particular temperature.
![]()
Vapour pressure of water P H2O is constant at a given temperature.
2) For solid ![]() liquid equilibrium there is only one temperature at which two phases can co-exist at a particular pressure. This temperature is known as melting point.
liquid equilibrium there is only one temperature at which two phases can co-exist at a particular pressure. This temperature is known as melting point.
![]()
exist at 273K at 1.0 atmosphere. At the melting point, the masses of the two phases remain constant provided no exchange of heat takes place between the system and the surroundings.
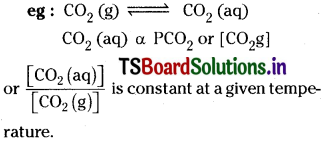
3) For dissolution of solids in liquids, the solubility (concentration of solid solute in solution) is constant at a given temperature.
![]()
Concentration of sugar in solution is constant at a given temperature.
4) For dissolution of gases in liquids the concentration of a gas in liquid, at a given temperature, is directly proportional to the pressure of the gas over the liquid.
Question 4.
What are the important features of equilibrium constant? Discuss any two applications of equilibrium constant.
Answer:
- The equilibrium constant has a definite value for every chemical reaction at a particular temperature. The value of equilibrium constant is independent of initial concentrations of reacting species.
- The value of equilibrium constant K changes with change in temperature.
- For a reversible reaction, the equilibrium constant for the backward reaction is inverse of the equilibrium constant for the forward reaction.
- The equilibrium constant is independent of the presence of catalyst.
- The equilibrium constant is expressed in terms of concentration, it has different units for different reactions.
- When addition of two equilibria leads to another equilibrium then the product of their equilibrium constants gives the equilibrium constant of the resultant equilibrium.
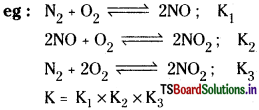
Applications of equilibrium constant:
1) Predicting of extent of reaction :
The magnitude of equilibrium constant tells us about the extent to which the reactants are converted into the products before the equilibrium is attained.
2) Predicting the direction of the reaction :
From the knowledge of equilibrium constant, it is possible to predict the direction in which the net reaction is taking place at a given concentrations or partial pressures of reactants and products.
If Qc > Kc the reaction taking place in backward direction is more.
If Qc < Kc the reaction taking place in forward direction is more.
If Qc = Kc the reaction is at equilibrium. No reaction taking place.
Question 5.
What is Le Chatelier’s principle? Discuss briefly the factors which can influence the equilibrium.
Answer:
Le Chatelier’s principle :
If a chemical reaction at equilibrium is subjected to a change in temperature, pressure or concentration that govern the chemical equilibrium, the equilibrium position shifts in the direction in which the change is reduced or nullified.
1) Effect of pressure :
Pressure is inversely proportional to volume. When pressure is increased the equilibrium shifts in such a direction where the decrease in volume is observed.
Similarly when pressure is decreased, the equilibrium shifts in such a direction where increase in volume is observed. This is applicable exclusively for gaseous reactions.
2) Effect of temperature:
When temperature is increased, equilibrium shift in such a direction where the temperature change is nullified or equilibrium shifts towards the direction in which temperature is decreased or heat is absorbed. That is endothermic reaction is favoured.
When temperature is decreased equilibrium shifts in such a direction where temperature is increased or heat is liberated. That is exothermic reaction is favoured.
3) Effect of concentration :
Increase in concentration of the reactants favours forward reaction and decrease in concentration of the reactants favours the backward reaction. When products are added to the equilibrium reaction the concentration of the products increases and reaction proceeds in backward direction. Removal of products leads to decrease in concentration of the products and leads to forward reaction.
![]()
Question 6.
Discuss the application of Le Chatelier’s principle for the industrial synthesis of Ammonia and sulphur trioxide. [Mar. ’18 (AP. TS) (AP, TS 16, 15]
Answer:
Application of Le Chatelier’s principle to
![]()
1) Effect of pressure :
Increase of external pressure on the reaction at equilibrium favours the reaction in the direction in which the volume or the number of molecules decreases.
In the above reaction of formation of ammonia forward reaction involves decrease in volume or number of molecules. So high pressure is favourable for the formation of ammonia. Hence ammonia is manufactured at 200 atm pressure.
2) Effect of temperature:
In a reversible reaction if the forward reaction is exothermic, the backward reaction will be endothermic. High temperature favours the endothermic reaction while low temperature favours the exothermic reaction. Since the formation of ammonia is exothermic reaction low temperature is favourable for the manufacture of ammonia. But at low temperature the reaction is slow. So an optimum temperature of about 725 – 775 K is used.
3) Effect of catalyst:
To increase the rate of formation of ammonia iron powder mixed with molybdenum powder is used as catalyst. Molybdenum acts as promoter to iron catalyst.
4) Effect of concentration :
Increase in concentrations of reactants increases the rate of forward reaction. Removal of the products from reaction mixture decreases the rate of backward reaction. So more ammonia can be obtained by increasing the concentration of N2 and H2 and removing the NH3 formed at regular intervals.
Optimum conditions for the synthesis of ammonia are
Pressure = 200 atm
Temperature = 725 – 775 K Catalyst = Fe
Promoter = Mo [Mar. ’18 (TS)]
Application of LeChatelier’s principle to
![]()
Effect of pressure:
Increase of external pressure on the reaction at equilibrium favours the reaction in the direction in which the volume or the number of molecules decreases.
Formation of SO3 in the above reaction involves decrease in volume. According to Le Chatelier’s principle high pressure is favourable for the formation of SO3. So SO3 is prepared at a pressure of 1.5 to 1.7 atmospheres.
Effect of temperature :
In a reversible reaction if the forward reaction is exothermic the backward reaction is endothermic. High temperatures are favourable for the endothermic reaction while the low temperatures are favourable for the exothermic reactions.
Since the formation of SO3 is exothermic reaction low temperatures are favourable. So SO3 is prepared at a temperature of about 673 K.
Effect of catalyst:
To increase the rate of formation of SO3 catalyst like V2O5 or platinised asbestos is used.
Effect of concentration :
Increase in the concentration of the reactants increase the rate of forward reaction. Removal of products from reaction mixture decreases the rate of backward reaction. So more SO3 can be obtained by increasing the concentrations of SO2 and O2 and by removing the SO3 formed at regular intervals.
Optimum conditions for the synthesis of ammonia are
Pressure : 1.5 – 1.7 atmospheres
Temperature: 673 K
Catalyst: V2O5 or Platinised asbestos.
Question 7.
Dihydrogen gas is obtained from natural gas by partial oxidation with steam as per the following endothermic reaction.
![]()
a) Write an expression for Kp for the above reaction.
b) How will the values of Kp and composition of equilibrium mixture be affected by (i) increasing the pressure (ii) increasing the temperature (lii) using a catalyst?
Answer:
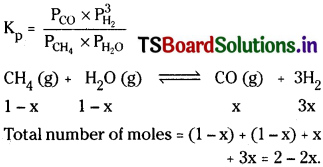
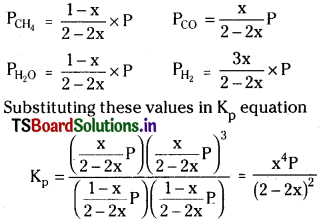
i) In the above equation the pressure term P is in the numerator. When pressure is increased to maintain the value of Kp constant the value in denominator should increase. Thus backward reaction takes place decreasing the amounts of products.
ii) Since the reaction is endothermic increasing the temperature favours the forward reaction forming more amounts of products.
iii) Catalyst does not influence the equilibrium mixture.
Question 8.
Describe the effect of:
a) addition of H2
b) addition of CH3OH
c) removal of CO
d) removal of CH3OH on the equilibrium of the reaction.
![]()
Answer:
a) According to Le Chatelier’s principle increase in the concentration of reactant increase the forward reaction.
So the addition of H2 results in the formation of more amount of CH3OH.
b) According to Le Chatelier’s principle increase in the concentration of the product shift the equilibrium in the backward direction. So the addition of CH3OH (product) results in the decrease in concentration and CH3OH and increase in the concentration of H2 and CO.
c) According to Le Chatelier’s principle the decrease in the concentration of reactant equilibrium shift in the backward direction. So removal of CO (reactant) the equilibrium shift in the backward direction. So formation of CH3OH decreases.
d) According to Le Chatelier’s principle the decrease in concentration of product shifts the equilibrium in the forward direction. So the removal of CH3OH (product) shift the equilibrium in the forward direction. Thus more amount of CH3OH is formed.
Question 9.
At 473K, equilibrium constant Kc for the decomposition of phosphorous pentachloride, PCl5, is 8.3 × 10-3. If the decomposition is depicted as:
![]()
a) Write an expression of Kc for the reaction
b) What is the value of Kc for the reverse reaction at the same temperature?
c) What would be effect on Kc if
(i) more PCl5 is added (ii) pressure is increased (iii) the temperature is increased.
Answer:
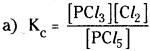
b) The value of Kc for the reverse reaction at the same temperature is the reciprocal of the Kc value of same reaction.
∴ Kc for reverse reaction = \(\frac{1}{8.3\times10^{-3}}\)
= 120.48.
c) i) If PCl5 is added equilibrium shifts in the forward direction but Kc does not change.
ii) If pressure is increased equilibrium shift in the backward direction but Kc does not change.
iii) The dissociation of PCl5 is endothermic reaction. So increase in temperatures causes more dissociation of PCl5. So Kc value increases.
![]()
Question 10.
Explain the concept of Bronsted acids and Bronsted bases. Illustrate the answer with suitable examples.
Answer:
Bronsted – Lowry theory :
Any substance that can lose a proton or protons is an acid.
Ex : HCl, H2SO4, H3PO4, CH3COOH.
Any substance that can gain a proton or protons is a base.
Ex : NH3, H2O, OH– etc.
When an acid react with base neutralization takes place. As per Bronsted – Lowry theory proton transfer from an acid to a base is called neutralization.
![]()
The above reaction is reversible. In this reaction,
- HCl donates proton to water. So it is a Bronsted acid.
- H2O gains the proton. So it is a Bronsted base.
- H3O+ donates a proton to Cl–. So it is Bronsted acid.
- Cl– can gain a proton. So it is a Bronsted base.
The acid base pair which differ by a single proton is said to be conjugate acidbase pair. In the above reaction HCl & Cl– and H3O+ and H2O are conjugate acidbase pairs.
Every Bronsted acid has its conjugate base and every Bronsted base has a conjugate protonic acid.
According to Bronsted – Lowry theory greater the tendency to donate proton stronger is the acid. Higher the tendency of the base to accept protons, stronger is the base. In the above reaction HCl gives proton easily than H3O+ So HCl is stronger acid than H2O. H2O accepts the proton more easily than Cl–. So H2O is a stronger base than NH3.
In all acidbase reactions, the reaction takes place in the direction of formation of weaker acid and a weaker base.
A stronger acid has a weaker conjugate base and a stronger base has weaker conjugate acid.
Advantages of Bronsted – Lowry theory :
- This theory explains the behaviour of acids and bases in both aqueous and non-aqueous solvents.
- It explains the behaviour of NH3, CaO, etc. as bases and CO2, SO2, etc. as acids.
Drawbacks of Bronsted – Lowry theory :
- Proton donation or acceptance happens only in the presence of other substances.
- It could not explain the behaviour of electron deficient molecules like AlCl3, BCl3, etc. as acids.
Question 11.
Explain Lewis acidbase theory with suitable example. Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/base.
a) OH– b) F– c) H+ d) BCl3
Answer:
Lewis acid:
The substance which can accept an electron pair to form a coordinate covalent bond is called Lewis acid.
Types of Lewis acids:
1) All +vely charged metal ions or cations act as Lewis acids.
ex : Ag+, Co+3, Cu+2, Fe+3, etc.
2) Electron deficient compounds whose central atom has an incomplete octet, possessing” an empty orbital acts as Lewis acid.
eg : BF3, BCl3, AlCl3, FeCl3.
3) Compounds in which central atom has vacant d’ orbitals and which can expand its octet.
eg : SiF4, SnCl4, SF4, TeF4, FeCl3.
4) Molecules having multiple bonds between atoms of different electrone-gativities.
eg : CO2, SO2, SO3, NO2, Cl2O7, P4O10.
5) Elements with an electron sextet.
eg: S, 0.
Lewis base :
The substance which donates electron pair to form a coordinate covalent bond is called Lewis base.
Types of Lewis bases :
1) All anions will act as Lewis bases.
eg : Cl– OH–, CN–, NH–2, F–, SCN–.
2) Molecules with one or two lone pairs on the central atom.
eg : H2O, NH3, R – OH, R – NH2, R – 0 – R, C5H5N.
3) Molecules with multiple bonds.
eg : CO, NO, HC ≡ CH, H2C = CH2
a) OH– is Lewis base,
b) F– is Lewis base,
c) H+ is Lewis acid,
d) BCl3 is Lewis acid.
![]()
Question 12.
What is degree of ionization in respect of weak acids and weak bases? Derive the relationship between degree of ionization (α) and ionization constant (Ka) for the weak acid HX.
Answer:
Degree of ionization :
It can be defined as the fraction of acid (a) base that undergoes ionization per one mole of acid or base. For strong acids or bases degree of ionization (α) is equal to T.
Let’s consider a weak acid HX with concentration ‘c’. It undergoes partial ionization.
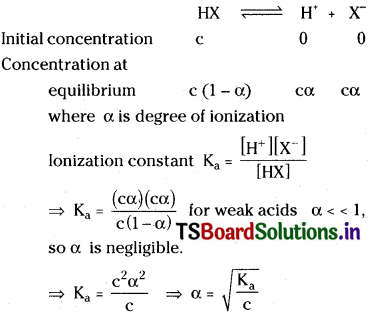
Question 13.
Define pH. What is buffer solution? Derive Henderson – Hasselbalch equation for calculating the pH of an acid buffer solution.
Answer:
The pH of a solution may be defined as negative logarithm of the activity of hydrogen ion (aH+)
pH = -log[H+]
Buffer solution :
A buffer solution is the solution which can resist the change in pH on addition of small amount of acid or base. The ability of a solution to resist change in pH on addition of small amount of acid or base is called buffer solution.
Henderson – Hasselbalch equation :
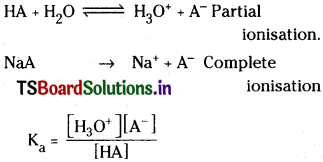
Since HA is weakly dissociated, the cone. HA ion solution can be taken equal to the initial cone, of the acid. Since NaA is completely dissociated, cone, of A– can be taken equal to the cone, of NaA (salt)
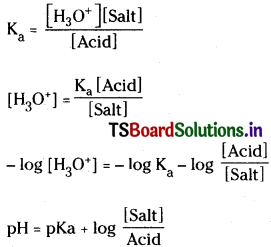
Question 14.
Explain the term “Hydrolysis of salts” with examples. Discuss the pH of the following types of salt solutions. [TS ’16]
i) Salts of weak acid and strong base.
ii) Salts of strong acitLand weak base.
Answer:
Hydrolysis of salts is the reverse process of neutralisation. In this process a salt react with water to form an acid and a base.
![]()
The fraction of the total salt that is hydrolysed at equilibrium is called degree or extent of hydrolysis. It is denoted by hy.
i) Salts of weak acid and strong base :
This type of salts produce alkaline solutions on hydrolysis.
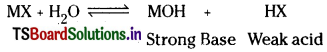
In solution MX and the strong base MOH completely ionise whereas acid HX being weak acid remains almost undissociated.
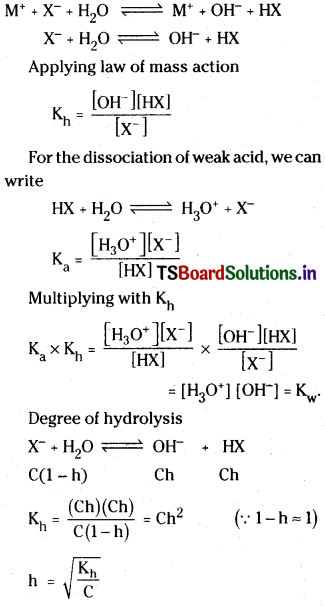
Hydronium ion conc. and pH
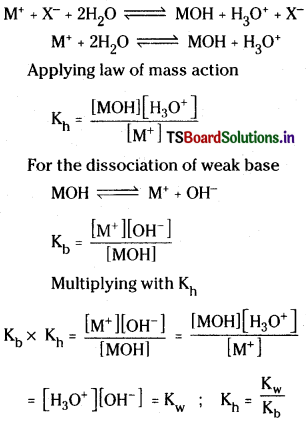
ii) Hydrolysis of salts of strong acid and weak base :
This type of salts produce acidic solutions on hydrolysis. For example,
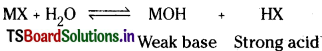
In solution MX and the strong acid HX completely ionises but the base MOH remains almost undissociated.
Degree of hydrolysis :
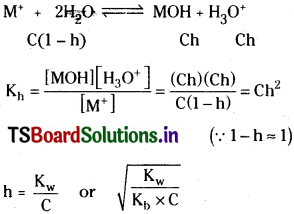
Hydronium ion concentration and pH

Question 15.
What is solubility product? Explain the common ion effect on solubility of ionic salts.
Answer:
Solubility product:
It can be defined as the product of concentration of cation and anion of a salt present in a saturated aqueous solution.
It is denoted with Ksp.
Units of Ksp = (Mole/Lt)n
where n = Total number of ions that can be given by salt per molecule on ionization.
Factors effecting Ksp :
Ksp can be effected by two factors only.
They are a) nature of substance b) temperature
Significance :
Solubility product is very important in preferential precipitation process in qualitative analysis.
The solubility of an electrolyte in water decreases by the addition of another electrolyte which has one ion common with the electrolyte.
For example the solubility of NaCl decreases on addition of HCl to solution of NaCl in water which is known as salting out of water.
Acetic acid is weak acid and it undergoes partial ionization as follows.
![]()
In case of such electrolytes there exists an equilibrium in between ions and molecular form. In such a condition the addition of electrolyte with common ions shifts the equilibrium in backward direction which causes the decrease in solubility.
Significance:
- Common ion effect is an important phenomenon in qualitative analysis.
- The common ion effect principle is also used in controlling the H+ ion concentration in buffer solution.
Question 16.
Write notes on
i) Common ion effect.
ii) The relation between Ksp and solubility (S) of a sparingly soluble salt BaSO4.
Answer:
i) Common ion effect:
The solubility of an electrolyte in water decreases by the addition of another electrolyte which has one ion common with the electrolyte.
For example the solubility of NaCl decreases on addition of HCl to solution of NaCl in water which is known as salting out of water.
Acetic acid is weak acid and it undergoes partial ionization as follows.
![]()
In case of such electrolytes there exists an equilibrium in between ions and molecular form. In such a condition the addition of electrolyte with common ions shifts the equilibrium in backward direction which causes the decrease in solubility.
Significance:
- Common ion effect is an important phenomenon in qualitative analysis.
- The common ion effect principle is also used in controlling the H+ ion concentration in bufferjsolution.
ii) The relation between Ksp and solubility (S) of a sparingly soluble salt BaSO4:
The sparing soluble salt like BaSO4, when added to water form saturated solution. There exists an equilibrium between undissolved solid and the ions in saturated solution.
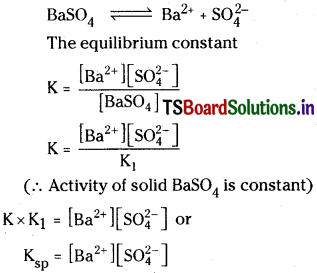
The new constant Ksp is called solubility product. If S moles lit-1 is the solubility of BaSO4.
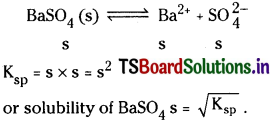
Numerical Problems
Question 1.
1 Mole of PCl5 is heated in a closed vessel of 1 litre capacity. At equilibrium 0.4 moles of chlorine is found. Calculate the equilibrium Constant.
Solution:
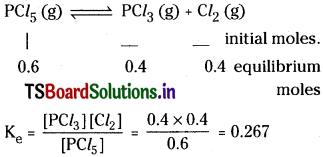
Question 2.
Nitrogen dioxide forms dinitrogen tetroxide according to the equation
![]()
when 0.1 mole of NO2 is added to a 1 litre flask at 25°C, the concentration changes so that at equilibrium [NO2] = 0.016M and [N2O4] = 0.042 M.
a) What is the value of the reaction Quotient before any reaction occurs.
b) What is the value of the equilibrium constant for the reaction.
Solution:
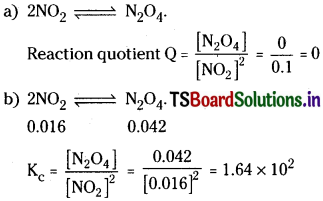
Question 3.
The equilibrium constant for the reaction:
![]()
is 6.0 × 10-2. At equilibrium, [H2] = 0.25 mol L-1 and [NO3] = 0.06 mol L-1.
Calculate the equilibrium concentration of N2.
Solution:
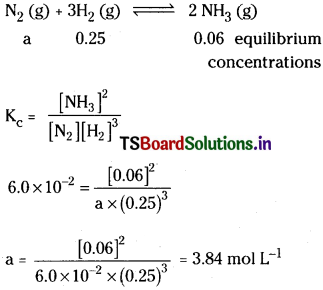
∴ The cone, of N2 at equilibrium = 3.84 mol L-1
![]()
Question 4.
At certain temperature, Kc for the reaction.
![]()
is 16. If initially one mole each of all the four gases are taken in one litre vessel, what are the equilibrium concentrations of NO and NO2?
Solution:
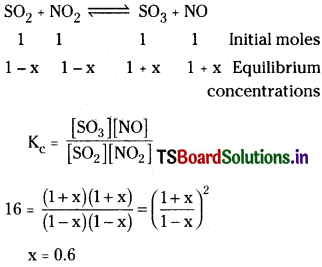
∴ At equilibrium [NO] = 1.6 mol L-1 and [NO2] = 0.4 mol L-1
Question 5.
Under certain conditions, the equilibrium constant for the decomposition of PCl5 (g) into PCl3 (g) and Cl2(g) is 0.0211 mol L-1. What are the equilibrium concentrations of PCl5, PCl3 and Cl2, if the initial concentration of PCl5 was 1.00 M ?
Solution:
![]()
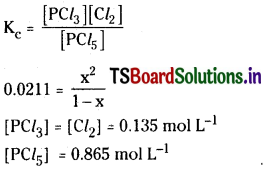
Question 6.
![]()
a 3 litre vessel contains 1, 2, 4 mole of A, B and C respectively predict the direction of reaction if
a) Kc for the reaction is 10
b) Kc for the reaction is 15
c) Kc for the reaction is 10.66
Solution:
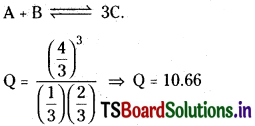
a) Kc for the reaction is 10
Q > Kc so backward reaction takes place.
b) Kc =15
Q < Kc so forward reaction takes place.
c) Kc = 10.66
Q = Kc Equilibrium is achieved.
Question 7.
A mixture of H2, N2 and NH3 with molar concentrations 5.0 × 10-3 mol L-1, 4.0 × 10-3 mol L-1 and 2.0 × 10-3 mol L-1 respectively was prepared and heated to 500K. The value of Kc for the reaction :
![]()
at this temperature is 60. Predict whether ammonia tends to form or decompose at this stage of concentration.
Solution:
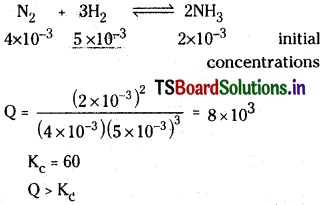
Since Q is greater than Kc, backward reaction takes place. So decomposition of NH3 takes place.
Question 8.
At 500 K, Kp value for the reaction
![]()
Find the value of Kp for each of following reactions at the same temperature.
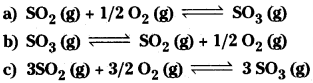
Solution:
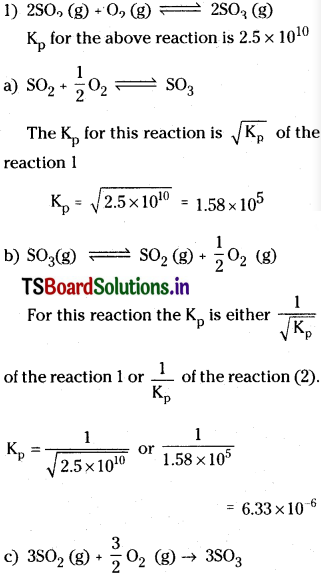
This reaction is obtained by multiplying the equation a) with three or by multiplying the equation (1) with 1.5.
So Kp for this reaction (Kp)³ of equation (a)
Kp = (1.58 × 105)³ = 3.944 × 1015
Question 9.
![]()
is 4.63 × 10-3 at 25°C.
a) What is the value of Kp at this temperature?
b) At 25°C, if the partial pressure of N2O4 (g) at equilibrium is 0.2 atm, calculate equilibrium pressure of NO2(g).
Solution:
![]()
a) Kp = Kc [RT]∆n
∆n for the above reaction = 2 – 1 = 1
∴ Kp = 4.63 × 10-3 × 0.0821 × 298
= 0.1132 atm.
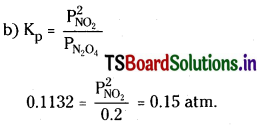
∴ Equilibriumpressure of NO2 = 0.15atm.
Question 10.
At 27°C, Kp value for the reversible reaction
![]()
0.65, calculate Kc.
Solution:
Kp = Kc[RT]∆n.

∴ Equilibrium constant Kc = 0.0264.
![]()
Question 11.
Kc for the reaction,
![]()
400K, find Kp.
Solution:

Question 12.
1 mole of A and 1 mole of Bare taken in a 5 litre flask, 0.5 mole of C is formed in the equilibrium of
![]()
What is molar concentration of each species if the reaction is carried with 2 mole of A, 1 mole of B in a 5 litre flask at the same temperature.
Solution:
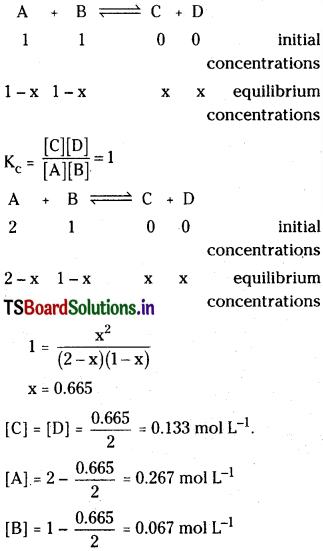
Question 13.
For the following reaction
![]()
of PCl5. 0.2 mole of PCl5 and 0.6 mole of Cl2 are taken in a 1 litre flask. If Kc = 0.2, predict the direction in which reaction proceeds.
Solution:
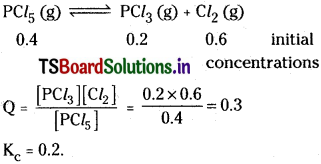
Q is greater than Kc. So reaction takes place in backward direction.
Question 14.
In an equilibrium
![]()
and B are mixed in a vessel at temperature T. The initial concentration of A was twice the initial concentration of B. After the attainment of equilibrium, concentration of C was thrice concentration of B, calculate Kc.
Solution:
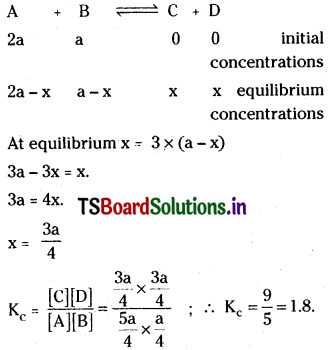
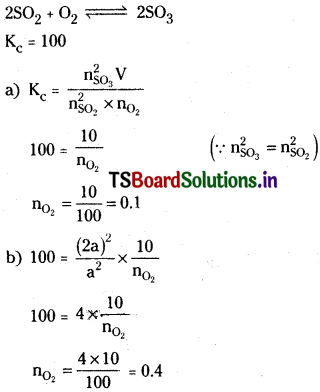
Question 15.
A mixture of SO2, SO3 and O2 gases are maintained at equilibrium in 10 litre flask at a temperature at which Kc for the reaction
![]()
is 100. At equilibrium
a) If no. of moles of SO3 and SO2 in flask are same, how many moles of O2 are present.
b) If no. of moles of SO3 in flask is twice the no. of moles SO2, how many moles of O2 are present.
Solution:
Question 16.
For ![]()
the equilibrium con centrations of A and B at a temperature are 15 mol L-1. When volume is doubled the reaction has equilibrium concentration of A as 10 mol L-1. Calculate
a) Kc
b) concentration of C in original equilibrium.
Solution:

If volume is doubled backward reaction is favoured since the number of moles of reactions are more.
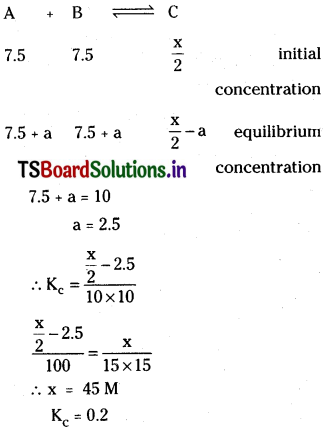
Question 17.
A vessel at 100K contains CO2 with a pressure of 0.5 atm. Some of the CO2 is converted into CO on addition of graphite. Calculate the value of K, if total pressure at equilibrium is 0.8 atm.
Solution:
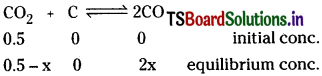
Total pressure at equilibrium = 0.8
equilibrium mixture contains CO and CO2.
∴ (0.5 + x) atm = 0.8 atm.
x = 0.3 atm
∴ Kp = \(\frac{(0.6)^2}{0.2}\) = 1.8atm.
![]()
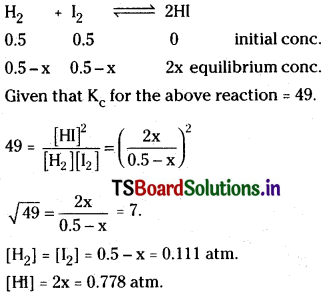
Question 18.
The Kp value for the reaction
![]()
If the initial pressure of H2 and I2 are 0.5 atm respectively, determine the partial pressure of each gases at equilibrium.
Solution:
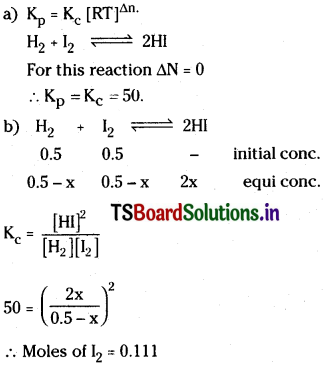
Question 19.
0.5 mol of H2 and 0.5 mole of I2 react in 10 litre flask at 448°C. The equilibrium constant Kc is 50 for
![]()
a) What is the value of Kp
b) Calculate mole of I2 at equilibrium.
Solution:
Question 20.
How much PCl5 must be added to a one little vessel at 250°C in order to obtain a concentration of 0.1 mole of Cl2 at equilibriuin. Kc for
![]()
is 0.0414 M.
Solution:
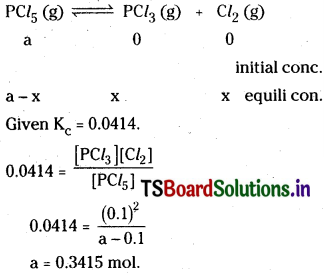
So 0.3415 mol of PCl5 should be added to the 1 litre flask to get 0.1 mol of Cl2 at equilibrium.
Question 21.
Kp for the reaction
![]()
a) Calculate Kc
b) Calculate ∆ G° value using Kc value.
Solution:
a) Kp for the reaction
![]()
is 1.64 × 10-4
Kp = Kc[RT]∆n
Here ∆n = 2 – 4 = -2
∴ Kp = Kc[RT]-2
1.64 × 10-4 = Kc[RT]-2
Kc = 1.64 × 10-4 × (0.0831 × 673)²
= 0.5129.5
b) ∆G° =-2.303 RT log Kc
= – 2.303 × 0.0831 × 673 log 0.5129.5
= 3873 J
Question 22.
Calculate pH of [TS ’15]
a) 10-3 M HCl
b) 10-3 MH2HO4
c) 10-6 M HNO3
d) 0.02 MH2 SO4
Answer:
a) pH = – log [H+] = – log [1 × 10-3] = 3
b) H2SO4 → 2H+ + SO2-4
Since 1 mole of H2SO4 gives 2 moles of H+
Concentration of H+ = 10-3 × 2 = 2 × 10-3
pH = -log [2 × 10-3] = 2.699
c) 10-6M HNO3
pH = – log [10-6] = 6
d) 0.02 M H2 SO4
[H+] = 0.04
(∵ 1 mole of H2SO4 gives 2 moles of H+)
pH = -log [4 × 10-2] = 1.4
![]()
Question 23.
Calculate the pH for
a) 0.001 M NaOH
b) 0.01 M Ca (OH)2
c) 0.0008M Ba (OH)2
d) 0.004 M NaOH
Solution:
pOH = – log [OH–]
pH = 14 – pOH.
a) pOH = – log [OH–] = – log [1 × 10-3 J = 3
pH = 14 – 3 = 11
b) 0.01 M Ca(OH)2
Cone, of [OH–] = 0.02
∴ 1 mol Ca(OH)2 gives 2 mol OH–
pOH = – log [2 × 10-2] = 1.6990
pH= 14- 1.6990= 12.3010
c) Ba(OH)2 → Ba2+ + 2OH–
0.0008 0.0016.
pOH = – log [1.6 × 10-3] = 2.7959
pH = 14 – 2.7959 = 11.2041.
d) NaOH -> Na+ + OH-
0.004 0.004
pOH = – log [4 × 10-3] = 2.3980
pH = 14-2.3980 = 11.6020
Question 24.
The pH of a solution is 3.6. Calculate HaO+ ion concentration.
Solution:
pH = 3.6
-log[H+] = 3.6
log [H+] = -3 – 0.6 + 1 – 1
[H+] = 2.5 × 10-4
Question 25.
The pH of a solution is 8.6. Calculate the OH– ion concentration.
Solution:
pH = 8.6
pOH = 5.4
– log [OH-] = 10-5.4
[OH–] = 10-6 × 100.6 = 100.6 × anti log 0.6
[OH–] = 3.98 × 100.6,
Question 26.
What is [H+] for a solution in which
a) pH = 3 b) pH = 4.75 c) = pH = 4.4?
Solution:
a) pH = 3
[H+] = 10-3
b) pH = 4.75
[H+] = 10-4.75
[H+] = 10-5 × 100.25
= 10-5 × antilog 0.25
= 1.778 × 10-5M.
c) pH = 4.4
– log [H+] = 4.4
[H+] = 10-4.4
= 100.25 × 100.6
= 1010-5 × antilog 0.6
= 3.981 × 1010-6 M.
![]()
Question 27.
A solution of 0.005 MH2SO4 is diluted 100 times. Calculate the pH of diluted solution.
Solution:
M1V1 = M2V2
M1 = 0.005 M2 = ?
V1 = 1 V2 = 100
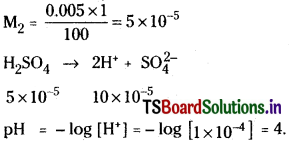
Question 28.
A solution of HCl has a pH = 3. If one ml of it is diluted to 1 litre, what will be the pH of the resulting solution?
Solution:
pH of HCl = 3
[H+] = 1 × 10-3.
1 m/ is diluted to 1000 ml.
Cone, after dilution = \(\frac{1\times10^{-3}}{100}\) = 1 × 10-6
∴ pH = 6.
Question 29.
What is the pH of HT8 M HCl? [TS ’15]
Solution:
[H+] = 10-8 + 10-7
= 0.1 × -7 + 1 × -7 = 1.1 × -7
pH = – log [1.1 × -7] = 6.96
Question 30.
Calculate the pH of the following basic solutions. [Mar. ’13]
a) [OH–] = 0.05 M
b) [OH–] = 2 × -4 M
Solution:
a) pOH = -log [5 × 10-2] = 1.3010
pH = 14 – 1.3010 = 12.6990
b) pOH = – log [2 × 10-4] =3.6979
pH = 14 – 3.6979 = 10.3010
Question 31.
2gof NaOH is dissolved in water to give 1 litresolution. What is the pH of the soiution?
Solution:
Molarity of NaOH = \(\frac{2}{40\times1}\)
NaOH -» Na+ + OH- 0.05 0.05
pOH = – log [OH–]
= – log [5 × 10-2]
= 1.3010 pH= 14- 1.3010 = 12.6990
Question 32.
Calculate the pH of the following solutions.
a) 0.37 g of Ca(OH)2 dissolved in water to give 500 ml solution
b) 0.3 g of NaOH dissolved in water to give 200 ml solution
c) 0.1825% HCl aqueous solution
d) 1 ml of 13.6 M HCl is diluted with water to give 1 litre solution.
Solution:
a) Molarity of Ca(OH)2
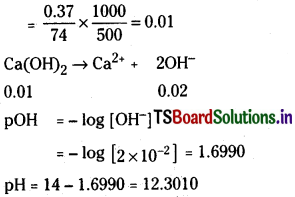
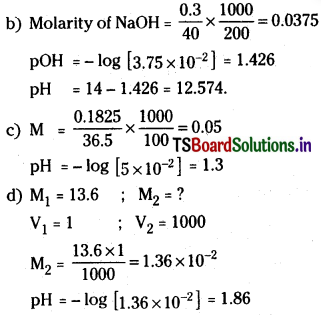
Question 33.
How many grams of NaOH are present in 100 ml solution if pH of die solution is 10?
Solution:
pH =10
pOH = 14 – 10 = 4.
[OH–] = 10-4
W = V N E
= \(\frac{100}{1000}\) × 10-4 × 40 = 4 × 10-4 gm
![]()
Question 34.
The value of 1^ is 9.55 x 10-14 at certain temperature. Calculate the pH of water at this temperature..
Solution:
[H+] [OH–] = Kw = 9.55 × 10-14
[H+] = \(\sqrt{9.55\times10^{-14}}\) = 3.09 × 10-7
pH = – log [3.09 × 10-7] = 6.51
Question 35.
Calculate the pH of 10-8 M NaOH.
Solution:
[OH–] = 10-8 + 10-7
= 0.1 × 10-7 + 1 × 10-7 = 1.1 × 10-7.
pOH = -log [1.1 × 10-7] = 6.96
pH = 14 – 6.96 = 7.04.
Question 36.
150 ml of 0.5 M HCl and 100 ml of 0.2 M HCl are mixed. Find die pH of the resulting solution.
Solution:

Question 37.
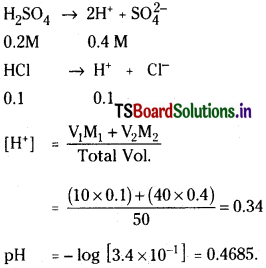
Calculate the pH of solution obtained by mixing 10 ml of 0.1 M HCl and 40 ml of 0.2 M H2SO4.
Solution:
Question 38.
100 ml of pH = 4 solution is mixed with 100 ml of pH = 6 solution. What is the pH of resulting solution?
Solution:
In first solution
[H+] = 10-4
and in the second solution
[H+] = 10-6
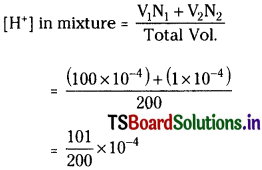
Approximately = 0.5 × 10-4 or 5 × 10-5
pH = – log [5× 10-5] = 4.3
Question 39.
Equal volumes of 0.5 M NaOH and 0.3 M KOH are mixed in an experiment. Find the POH and pH of the resulting solution.
Solution:
Cone, of the mixed solution of NaOH and KOH.

pOH = – log [4 × 10-1] =0.3980
pH = 14-0.3980 = 13.6021.
Question 40.
60 ml of 1 M HCl is mixed with 40 ml of 1M NaOH. What is the pH of resultant solution?
Solution:
HCl + NaOH → NaCl + H2O
Here acid is neutralised with base
So the concentration of acid

Question 41.
Calculate the pH of a solution which contains 100 ml of 0.1 M HCl and 9.9 ml of 1.0 M NaOH.
Solution:
Here 100 ml of 0.1 M HCl
meq = 100 × 0.1 = 10
9.9 ml of 1.0 M NaOH.
meq = 9.9 × 1 = 9.9 meq
meq of acid left = 10 – 9.9 = 0.1

Question 42.
What will be the resultant pH when 200 ml of an aqueous-solution of HCl having PH = 2 is mixed with 300 ml of an aqueous solution of NaOH having pH = 12?
Solution:
Concentration of HCl = 10-2
pH of NaOH = 12
pOH of NaOH = 14 – 12 = 2.
Concentration of NaOH = 10-2.
200 ml of 10-2 HCl is mixed with 300 mL of 10-2 NaOH. Since NaOH is more the resultant solution is basic.
Concentration of NaOH left
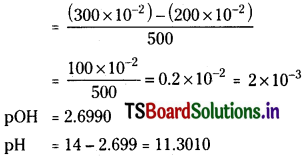
Question 43.
50 ml of 0.2 M HCl is added to 30 ml of 0.1 M KOH solution. Find the pH of the solution.
Solution:
Since acid is more than base the resultant solution is acidic.
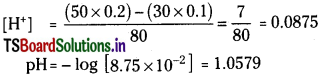
Question 44.
40 ml of 0.2 MHNO3 when reacted with 60 ml of 0.3 M NaOH, gave a mixed solution. What is the pH of the resulting solution?
Solution:
Since base is more than acid the resultant solution is basic.
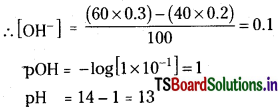
Question 45.
50 ml of 0.1 M H2SO4 were added to 100 ml of 0.2 M HNO3. Then the solution is diluted to 300 ml. What is the pH of the solution?
Solution:

Concentration of H+ in the resultant solution
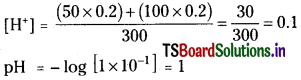
Question 46.
What is the Kw Rvalue in an aqueous solution of pKw = 13.725?
Solution:
pKw = -logKw
Kw = 1.884 × 10-14
![]()
Question 47.
The ionic product of water at 80°C is 2.44 × 10-13. What are the concentrations of hydronium ion and the hydroxide in pure water at 80°C?
Solution:
Ionic product of water at 80°C = 2.44 × 10-13
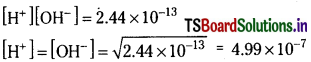
Question 48.
The ionization constant for water is 2.9 × 10-14 at 40°C. Calculate [H3O+], [OH], pH and pOH for pure water at 40°C.
Solution:
The ionisation constant for water at 40°C
= 2.9 × 10-14
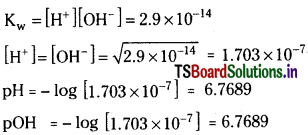
Question 49.
Calculate the pH of
a) 0.002 M acetic acid having 2.3% dissociation.
b) 0.002 M NH4OH having 2.3% dissociation.
Solution:

[H+] = Cα = 0.002 × 0.023 = 4.6 × 10-5
pH = – log [4.6 × 10-5] = 4.3372.
b) [OH–]= 4.6 × 10-5
pOH = 4.3372
pH = 14-4.3372 = 9.66.
Question 50.
Calculate Ka of acetic acid from equilibrium concentration given below.
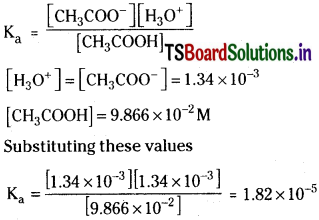
[H3O+] = [CH3COO–] = 1.34 × 10-3 M,
[CH3COOH] = 9.866 × 10-2 M
Solution:
Question 51.
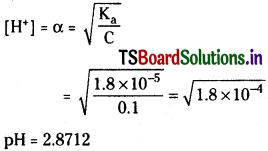
Calculate pH of 0.1 M acetic acid having K = 1.8 × 10-5.
Solution:
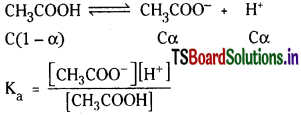
Ka = \(\frac{(C \alpha)(C \alpha)}{C(1-\alpha)}\)
or Ka = Cα² (a in the denominator can be neglected)
Question 52.
The pH of 0.1 M solution of weak mono protic acid is 4.0. Calculate its [H+] and Ka.
Solution:
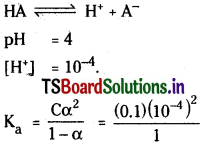
Neglecting a in the denominator.
Ka = 10-7
Question 53.
Ka of 0.02 M CH³ COOH is 1.8 × 10-5. Calculate
a) [H3O+] b) % ionization c) pH
Solution:
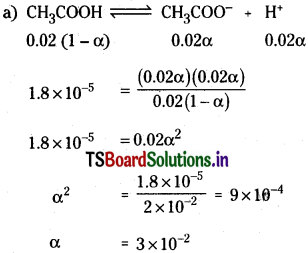
[H3O+] = Cα = 0.02 × 3 × 10-2 = 6 × 10-4
% Ionisation = 3 x 10“2 x 100 = 3%.
pH = – log [H3O+]
= -log [6 × 10-4]
= 3.22.
Question 54.
Calculate the pH of 0.01 M solution of CH3COOH. Ka for CH3COOH at 298 K is 1.8 × 10-5.
Solution:
![]()
α = 4.1 × 10-2
∴ [H3O+] = 0.01 × 4.1 × 10-2 = 4.1 × 10-4
pH = -log [H3O+]
s = -log (4.1 × 10-4) = 3.38.
Question 55.
The pH of 0.1 M solution of an organic acid is 4.0. Calculate the dissociation constant of the acid.
Solution:
pH of the solution = 4
∴ [H3O+] = 10-4
Organic acid HX ionises
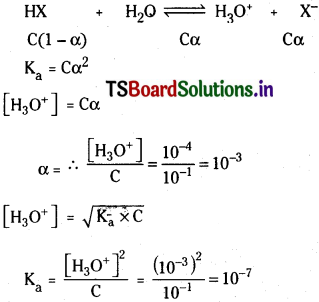
Question 56.
The ionization constants of HF, H COOH and HCN at 298 K are 6.8 × 10-4, 1.8 × 10-4 and 4.8 × 10-9 respectively. Calculate the ionization constants of the corresponding conjugate base.
Solution:
Kw = Ka × Kb
HF → H+ + F–
Ionisation constant of F–
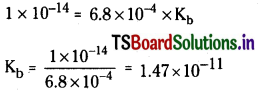
Similarly for

Question 57.
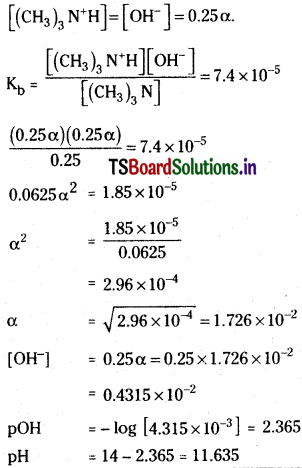
Find the concentration of hydroxide ion in a 0.25 M solution of trimethylamine, a weak base.

Solution:
Ionisation constant of weak base Kb
Suppose α is the degree of association of the base
At equilibrium
[trimethyl amine] = 0.25 (1 – α) = 0.25 M.
Question 58.
The 0.005 M monobasic acid has a pH of 5. What is the extent of ionization ?
Solution:
pH of the solution = 5
[H+] = 10-5
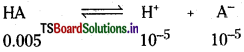
Question 59.
50 ml of 0.1 M NH4OH, 25 ml of 2 M NH4Cl were used to make a buffer. What is the pH if pKa is 4.8?
Solution:
For a basic buffer solution
pOH = pKb + log\(\frac{M_1V_1}{M_2V_2}\)
M1 = moles of salt 2
M2 = moles of base 0.1
V1 = Volume of salt 25 ml
V2 = Volume of base 50
pOH = 4.8 + log\(\frac{2\times25}{0.1\times50}\)
= 4.8 + log 10 = 5.8
pH = 14 – 5.8 = 8.2
![]()
Question 60.
The pH of a buffer prepared by mixing 50 ml of 0.2 M CH3COOH and 25 ml of CH3COONa is 4.8. If the pKa is 4.8, what is the strength of CH3COONa?
Solution:
For acidic buffer solution
pH = pKa + log\(\frac{[Salt]}{[Acid]}\) = pKa + log\(\frac{M_1V_1}{M_2V_2}\)
M1 = moles of salt ?
M2 = moles of base 0.2
V1 = Volume of salt 25
V2 = Volume of acid 50
pH = 4.8 pKa = 4.8
When the [Salt] = [Acid] pH = pH.
∴ M1 × 25 = 0.2 × 50
M1 = \(\frac{2.5\times50}{25}\) = 0.4 M.
Question 61.
50 ml of 0.1 M sodium acetate, 25 ml of 0.2 M acetic acid were added together to form the buffer solution. pKa of CH3COOH is 4.8. Find the pH of the solution.
Solution:
For acidic buffer
pH = pKa + log\(\frac{[Salt]}{[Acid]}\)
[Salt] =VoIume of Salt x Moles of Salt = M1V1
[Acid] = Volume of acid x Moles of acid = M2V2
pH = 48 + l0g\(\frac{50\times0.1}{25\times0.2}\)
∴ pH = 4.8
Question 62.
When 20 ml of 0.1 M NH4OH are added to 20 ml of MNH4Cl solution, the pH of the buffer formed is 8.2. What is the pKb of NH4OH?
Solution:
For basic buffer
pOH – PKb + log\(\frac{[Salt]}{[Acid]}\)
Given the pH = 8.2
∴ pOH = 14 – 8.2 = 5.8.
5.8 pKb + l0g\(\frac{20\times1}{20\times0.1}\)
5.8= pKb + log 10
pKb = 5.8 – 1 = 4.8
Question 63.
One litre of buffer solution contains 0.1 mole of acetic acid add 1 mole of sodium acetate. Find its pH if pKa of CH3COOH is 4.8.
Solution:
For acidic buffer
pH = pKa + log\(\frac{[Salt]}{[Acid]}\)
= 4.8 + log\(\frac{1}{0.1}\)
= 4.8 + log 10 = 4.8 + 1 = 5.8.
Question 64.
50 ml of 1 M CH3COOH solution, when added to 50 ml of 0.5 M NaOH gives a solution with a pH value ‘X’. Find the value of ‘X’, pKa of acetic acid is 4.8.
Solution:
CH3COOH + NaOH → CH3COONa + H2O
50 ml of 0.5 M NaOH neutralises the acetic acid
V1N1 = V2N2
50 × 0.5= V2 × 1
∴ V2 = \(\frac{50\times0.5}{1}\) = 50
So 50% acetic acid is neutralised.
Therefore the resulting solution contains equal amounts of acetic acid and sodium acetate. So
pH = 4.8 + log \(\frac{[Salt]}{[Acid]}\) = 4.8
![]()
Question 65.
The solubility product of Ag Cl is 1.6 × 10-10 mol²/ L². What is its solubility?
Solution:
Question 66.
The solubility product of Zr (OH)2 is 4.5 × 10-17 mol³ L-3. What is solubility?
Solution:
Question 67.
The solubility of Ag2 CrO4 is 1.3 × 10-4 mol L-1. What is the solubility product?
Solution:
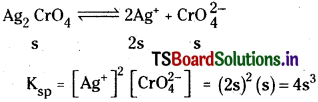
Solubility = 1.3 × 10-4
Ksp = 4s³ = 4 × (1.3 × 10-4)³
= 8.78 × 10-12
Question 68.
The solubility of A2B = 2 × 10-3 mol L-1. What is solubility product?
Solution:
![]()
Ksp = [A]² [B]
= (2s)² (s) = 4s²
Solubility s = 2 × 10-3 mol L-1
Ksp = 4(2 × 10-3)³= 3.2 × 10-10
Question 69.
The solubility product of a salt AB = 10-10 mol² L-2. What is solubility?
Solution:
![]()
Ksp = [A] [B] = s × s = s²
s² = 10-10
s = 10-5 mol L-1.
Question 70.
PQ and RS2 are two sparingly soluble salts. Their solubility products are equal and each equal to 4.0 x 10-18. Which salt is more soluble?
Solution:
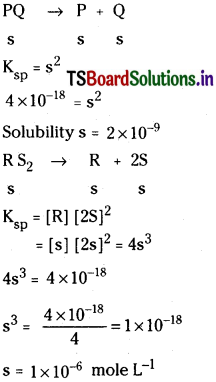
∴ RS2 is more soluble.
Question 71.
In a 0.1 M solution, acetic acid is 1.34% ionized. Calculate [H+], [CH3COO–] and [CH3COOH] in the solution and calculate Ka of acetic acid.
Solution:
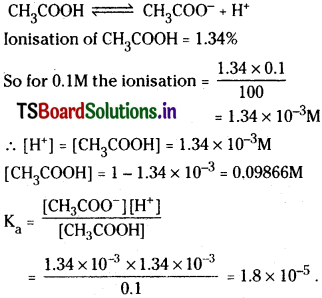
Additional Questions & Answers
Question 1.
PCl5, PCl3 and Cl2 are at equilibrium at 500 K and having concentration 1.59M PCl3, 1.59M Cl2 and 1.41 M PCl5. Calculate Kc for the reaction,
![]()
Answer:
The equilibrium constant Kc for the above reaction can be written as,
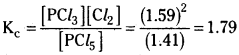
Question 2.
The value of ∆GΘ for the phosphorylation of glucose in glycolysis is 13.8 kJ/mol. Find the value of Kc at 298 K.
Answer:
∆GΘ = 13.8 kJ / mol = 13.8 × 10³J/ mol
Also, ∆GΘ = – RT InKc
Hence, In Kc = -13.8 × 10³J/mol (8.314 J mob-1 K-1 × 298 K)
In Kc = -5.569
Kc = e-5.569
Kc = 3.81 × 10-3
Question 3.
What will be the conjugate bases for the following Bronsted acids: HF, H2SO4 and HCO2-3?
Answer:
The conjugate bases should have one proton less in each case and therefore the corresponding conjugate bases are: F–, HSO–4 and CO2-3 respectively.
Question 4.
Write the conjugate acids for the following Bronsted bases: NH–2, NH3 and HCOO–.
Answer:
The conjugate acid should have one extra proton in each case and therefore the corresponding conjugate acids are: NH3, NH+4 and HCOOH respectively.
![]()
Question 5.
The species: H2O, HCO–3, HSO–4 and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and conjugate base.
Answer:
The answer is given in the following table:
| Species | Conjugate acid | Conjugate base |
| H2O | H3O+ | OH– |
| HCO–3 | H2CO3 | CO2-3 |
| HSO–4 | H2SO4 | SO2-4 |
| NH3 | NH+4 | NH–2 |
Question 6.
The concentration of hydrogen ion in a sample of soft drink is 3.8 × 10-3M. What is its pH?
Answer:
pH = – log[3.8 × 10-3]
= -{log[3.8]+log[10-3]}
= – {(0.58) + (-3.0)} = – {-2.42} = 2.42
Therefore, the pH of the soft drink is 2.42 and it can be inferred that it is acidic.
Question 7.
Calculate pH of a 1.0 × 10-8 M solution of HCl.
Answer:
![]()
Kw = [OH–][H3O+] = 10-14
Let, x = [OH–] = [H3O+] from H2O. The H3O+ concentration is generated (i) from the ionization of HC1 dissolved i.e.,
![]()
and (ii) from ionization of H2O. In these very dilute solutions, both sources of H3O+ must be considered:
[H3O+] = 10-8 + x
Kw = (10-8 + x)(x) = 10-14
or x² + 10-8 x – 10-14 = 0
[OH–] = x = 9.5 × 10-8
So, pOH = 7.02 and pH = 6.98
![]()
Question 8.
Calculate the solubility of A2X3 in pure water, assuming that neither kind of ion reacts with water. The solubility product of A2X3, Ksp = 1.1 × 10-23
Answer:
![]()
Ksp = [A3+]² [X2-]³ = 1.1 × 10-23
If S = solubility of A2X3, then
[A3+] = 2S; [X²] = 3S
therefore, Ksp = (2S)²(3S)³ = 108S5
= 1.1 × 10-23
thus, S5 = 1 × 10-25
S = 1.0 × 10-5 mol/L.