Telangana SCERT TS 10th Class Physical Science Study Material Pdf 6th Lesson Structure of Atom Textbook Questions and Answers.
TS 10th Class Physical Science 6th Lesson Questions and Answers Structure of Atom
Improve Your Learning
I. Reflections on concepts
Question 1.
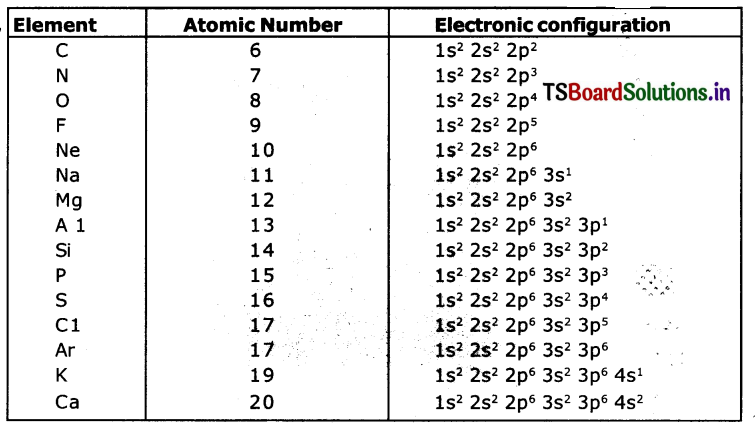
What information does the electronic configuration of an atom provide?
Answer:
- The distribution of electrons in shells, sub-shells arid orbitals in an atom is ‘known as electronic configuration.
- The distribution of electrons In various atomic orbitals provides an understanding of the electronic behaviour of the atom and in turn its reactivity.
- The short-hand notation is as shown below.
Question 2.
Rainbow is an example for continuous spectrum – explain.
Answer:
- A rainbow is a natural spectrum appearing in the sky while it is drizzling and the sun is in the east or west.
- It is caused by dispersion of sunlight by tiny water droplets present in atmosphere. It consists of 7 colours.
- In a rainbow, there are no sharp boundaries in between colours.
- Such a spectrum in which there are no sharp boundaries in between colours is known as continuous spectrum.
- So, rainbow is also a continuous spectrum.
Question 3.
What is an orbital? How is it different from Bohr’s orbit?
Answer:
The region or space around the nucleus where the probability of- finding the electron is maximum is called an orbital.
- Bohr’s orbit has a definite boundary and fixed energy at different distances from the nucleus. They are circular In shape.
- OrbitaIs have no definite boundary. It Is a region where we find maximum possibility of electrons. The shape of each orbital is different.
- Bohr’s orbit can accommodate maximum of (2n2) electrons ¡n it, but each orbital can accommodate only 2 electrons.
Question 4.
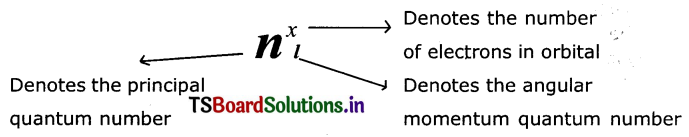
Explain the significance of three Quantum numbers in predicting the positions of an electron in an atom.
Answer:
Each electron In an atom Is described by a set of three quantum numbers n, l, and ml.
(1) PrincIpal Quantum number (n): The principal quantum number is used to describe the size and energy of the main shell. It Is denoted by ‘n’. ‘n’ has positive It is use integer values of 1,2,3.
It is used to know the number of orbitals (n2) and electrons in an orbit. (2n2).
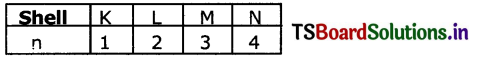
As ‘n’ increases the shells become larger and the electrons in those shells are farther from the nucleus.
2. The angular-momentum quantum number (l) :
from ‘0’ to n – 1, for each value of ‘n’. Each ‘l’ value represented one sub-shell. It is used to describe the shape of an orbit.

3. The magnetic quantum number (ml): The magnetic quantum number (ml) has integer values between – l and + l including zero.
If l= 0, the possible ml, value is l
l=1, the possible ml, value is- 1,0 and 1.
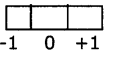
Thus for a certain value of 1, there are (2l + 1) integer values of ml.
These values describe the orientation of the orbital in space relative to the other orbitals in the atom,
Eg: When l = 1, (2l + 1) = 3, that means ni has 3 values namely – 1, 0, 1 or three p orbitals, with different orientations along X, Y, Z axes, labelled as px, py and pz orbitals. Predicting the position of an electron in an atom: If the values of n, l and m1 are 2, 1, -1 respectively the electron is present in 2px orbital in L shell.
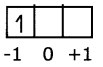
Question 5.
What is nlx method? How It is useful?
Answer:
The shorthand notation consists of the principal energy level (n value) the letter representing sub-level (l value) and the number of electrons In the sub-shell is written as superscript nlx It is useful in writing electron configurations of elements. For example in hydrogen (H), the set of quantum numbers Is n = 1,l = 0, ml= 0, ms= 1/2 or -1/2
The electronic configuration is
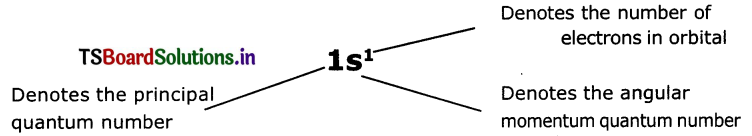
![]()
Question 6.
Which electronic shell Is a higher energy level K or L?
Answer:
- L shell has higher energy because according to Bohrs theory the shell which Is closer to nucleus has lower energy and the shell which is away from the nucleus has higher energy.
- K is closer to nucleus. So it has lower energy than L-shell.
Question 7.
What Is absorption spectrum?
Answer:
Absorption spectrum: The spectrum formed by the absorption of energy when electron jumps from lower energy level to higher energy level is called absorption Spectrum. It contains dark lines on bright background.
Question 8.
What is emission spectrum?
Answer:
Emission spectrum is the spectrum of frequencies of electromagnetic radiation due to an atom’s electron making a transition from a high-energy state to low energy state.
Application Of Concepts
Question 1.
Answer the following questions.
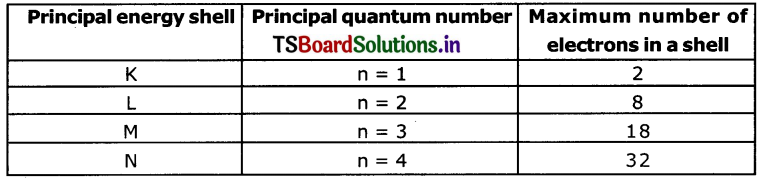
a. How many maximum number of electrons that can be accommodated In a principal energy level?
Answer:
The maximum number of electrons that can be accommodated in a principal energy shell is given by the rule 2n2, where ‘n’ is the principal quantum number.
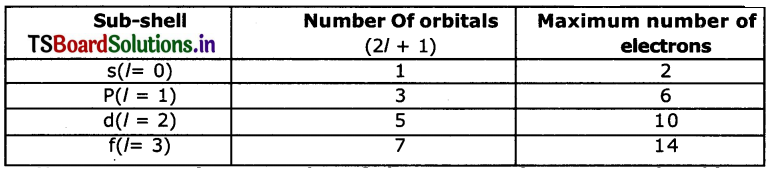
b. How many maximum numbers of electrons that can be accommodated In a sub-shell?
Answer:
- each subshell holds a maximum of twice as many electrons as the number of orbitals in the sub-shell.
- The maximum number of electrons that can occupy various sub-shells is
c. How many maximum numbers of electrons can be accommodated In an orbital?
Answer:
The maximum number of electrons that can be accommodated In an orbital is 2.
d. How many sub-shells are present In a principal energy shell?
Answer:
The number of sub-shells present in a principal energy shell is equal to principal quantum number ‘n’. Eg:
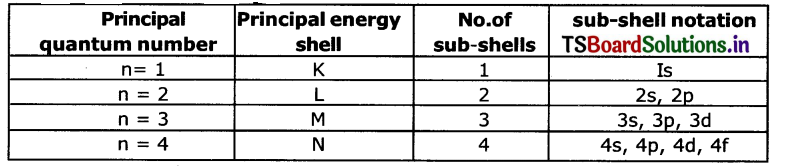
e. How many spin orientations are possible for an electron in an orbital?
Answer:
Two spin orientations are possible for an electron in an orbital, one clockwise and the other anticlockwise spin. There are represented by + 1/2 and – 1/2.
![]()
Question 2.
In an atom the number of electrons In M-shell is equal to the number of electrons in the K and L shells. Answer the following questions.
(a) Which is the outermost shell?
(b)How many electrons are there In its outermost shell?
(C) What is the atomic number of element?
(d) Write the electronic configuration of the element.
Answer:
No. of electrons in M’ shell Is equal to number of electrons In the K and L shells.
∴ No. of electrons n M shell = 2 + 8 = 10
Total electrons in the given atom = 2(K) + 8(L) + 10 (M) = 20
The electronic configuration Is = Is22s22p63s23p64s23d2
(a) M shell is the outermost shell.
(b) There are ‘2’ electrons in the outermost shell.
(c) The atomic number of the element is 20.
(d) Electronic configuration of the element: Is22s22p63s23p44s2
Question 3.
How many elliptical orbits are there in third Bohr’s orbit?
Answer:
- To explain the splitting of line spectra, Sommerfeld modified Bohr’s atomic model by adding elliptical orbits.
- RetaIning the first of Bohr’s circular orbit as such, Sommerfeld added two elliptical orbits to Bohr’s third orbit.
Question 4.
Following orbital diagram shows the electron configuration of nitrogen atom. Which rule does not support this? N (Z = 7)
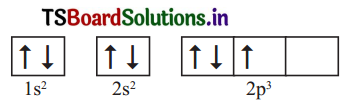
Answer:
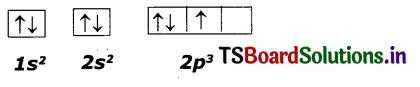
This electronic configuration of nitrogen is not correct.
It does not support Hund’s rule which states, the orbitals of equal energy are occupied by one electron each before pairing of electrons starts.
So the correct electron configuration is:
N (Z=7)
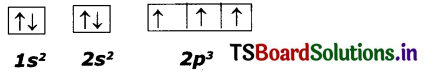
Question 5.
(i) An electron in an atom has the following set of four quantum numbers. To which orbital does it belong?
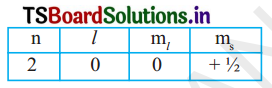
(ii) Write the four quantum numbers for is’ 1S1 electron.
Answer:
(i) Given n = 2 and l= 0 represent’s’orbital so the orbital is 2s and s= + ½ so by n1x method it is 2s1.
(ii) The four quantum numbers for Is1’ electron is

Question 6.
The wavelength of a radio wave is 1.0 m. Find its frequency.
Answer:
Given:
Wavelength λ = 1.0 m
We know that velocity of light c = vλ
Where c = 3 x 108 m/s
∴ 3 × 108 = v.1
v= 3 x 108
So, frequency of wave = 3 x 108 Hertz
Question 7.
Which rule Is violated in the electronic configuration is° 252 2p4?
Answer:
In the above electronic configuration Aufbau principle is violated.
(n+ 1) valueof isis 1+0 = 1
(n + 1)valueof2sis2 +0 = 2
Here electrons are filled in 2s Orbital, without completing is orbital which has lower (n + 1) value than 2s. Hence Aufbau principle is violated.
![]()
Question 8.
Write the four quantum numbers for the differentiating electron of sodiun (Na atom).
Answer:
(i) Electron configuration of sodium (Z = 11)
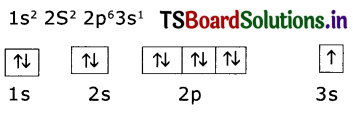
(ii) The differentiating electron is in 3s1. The four quantum numbers of this electron are

Question 8.
Collect the information regarding wavelengths and corresponding frequencies of three primary colours red, blue and green.
Answer:
| Colour | Frequency (THz) | Wavelength (nm) |
| Red | 400 – 494 | 620-750 |
| Blue | 606- 668 | 450-495 |
| Green | 526-606 | 495-570 |
Red colour has more wavelength among the seven colours of VIBGYOR. So it can be invisible from larger distances also. So it is used In signal lights.
Multiple choice questions
Question 1.
An emission spectrum consists of bright spectral lines on a dark background. Which one of the following does not correspond to the bright spectral lines? [ ]
(A) Frequency of emitted radiation
(B) Wavelength of emitted radiation
(C) Energy of emitted radiations
(D) Velocity of light
Answer:
(D) Velocity of light
Question 2.
The maximum number of electrons that can be accommodated in the L – shell of an atom is …………………. .[ ]
(A) 2
(B) 4
(C) 8
(D) 16
Answer:
(C) 8
Question 3.
If l=l for an atom then the number of orbitals in its sub-shell is ……………………… .[ ]
(A) 1
(B) 2
(C) 3
(D) 0
Answer:
(C) 3
Question 4.
The quantum number which explains about size and energy of the orbit or shell is: …………………….. . [ ]
(A) n
(B) l
(C) ml
(D) ms
Answer:
(A) n
![]()
Suggested Projects
Question 1.
Collect the information of historical development of atomic theory.
Answer:
The first atomic theory was proposed by. John Dalton based on law of conservation of mass and law of constant proportions.
The important postulates of his theory are:
- Atoms were indivisible.
- Atoms of an element are all identical to each other and different from the atoms of other elements.
Later on various experiments conducted by Thomson, Goldstein etc. proved that atom is divisible and consists of sub-atomic particles like electrons, protons and neutrons. Based on this J.J.
Thomson proposed a model of atom in 1898. According to Thomson,
- An atom is considered to be a sphere of uniform positive charge and electrons are embedded in It.
- The total mass of the atom is considered to be uniformly distributed throughout the atom.
- The negative and the positive charges are supposed to be balanced out and the atom as a whole is electrically neutral.
This model is also called as plum pudding model or watermelon model. Thomson’s student Ernest Rutherford conducted alpha particle scattering experiment got the results which were not in favour of Thomson’s model.
Based on his experiment, Rutherford proposed a model of atom. According to him,
- All the positively charged material In an atom formed a small dense Centre called the nucleus of the atom. The electrons were not a part of nucleus.
- Negatively charged electrons revolve around the nucleus is well-defined orbits like planets revolve around the sun.
- The size of nucleus is very small as compared to the size of the atom.
This model could not account for stability of atom, as revolving electron must lose energy and eventually crash into the nucleus, as a result matter would not exist in the it he form that we see it now. In 1913 Nells Bohr proposed another model to overcome Rutherford’s defect According to Bohr.
- The electrons revolve around the nuleus In a discrete orbits called stationary, orbits.
- While revolving in these discrete orbits the electrons do not radiate enery and this helps that the electrons do not crash into the nucleus.
- Theseorbitsorshellsare represented by K, L, M, N, ……………………………. or the numbers 1,2,3, …………………….
This model could not predict the spectra of atoms later Bohr along with Sommerfeld proposed the Bohr-Sommerfeld model of atom to account for the spectra of atoms.
Question 2.
Collect the information about the scientists who developed the atomic theories.
Answer:
(a) John Dalton
- Chemist John Dalton was born on September 6, 1766, In Eagles field, England.
- During his early career, he identified the hereditary nature of red-green colour blindness.
- In 1803, he revealed the concept of Daltons’ law of partial pressures.
- Also in the 1800s, he was the first scientist to explain the behaviour of atoms in terms of the measurement weight.
- During the early 1800s, Dalton also postulated a law of thermal expansion that illustrated the heating and cooling reaction of gases to expansion and compression.
- After suffering a second stroke, Dalton died quietly on the evening of July 26, 1844, at his home in Manchester England.

(b) J.J. Thomson:
- J.J. Thomson was born on December 18, 1856, in Cheetham Hill, England and went on to attend Trinity College at Cambridge, where he would come to head the Cavendish Laboratory.
- His research In Cathode rays led to the discovery of electrons and he pursued further innovations In atomic structure exploration.
- Thomson won the 1906 Nobel Prize in Physics, among many accolades.
- He died on August 30, 1940.

(c) Ernest Rutherford:
- Chemist and Physicist Ernest Rutherford was born on August 30, 1871, n Spring Grove, New Zealand.
- A pioneer of Nuclear Physics and the first to split the atom, Rutherford was awarded the 1908 Nobel Prize in Chemistry for his theory of atomic structure.
- He was the fourth of 12 children and second son. His father, James, had little education and struggled to support the large family on a flax-miller’s income.
- Ernest’s Mother, Martha, worked as a school teacher. She believed that knowledge was power, and placed a strong emphasis on her children’s education.
- Together, Rutherford and Thomson studied the effects of X-rays on the conductivity of gases, resulting Is a paper about dividing atoms and molecules into ions.

6. Famous as the “Father of the Nuclear Age”, Rutherford died in Cambridge, England, on October 19, 1937.
![]()
(d) Neils Bohr:
- (1) Born on October 7, 1885, In Copen Hagan, Denmark, Nells Bohr went on to become an accomplished physicist who came up with a revolutionary theory on atomic structures and radiation emission.
- (2) He won the 1922 Nobel Prize in physics for his ideas and years later, after working on the Manhattan project in the United States, called for responsible and peaceful applications of atomic energy across the world.
- (3) In 1957, Bohr received the “Atoms for Peace Award” for his trail-blazing theories and effects to use atomic energy wisely.
- (4) After having a stroke, he died on November 18, 1962, in Copen hagen. Bohr’s son Aage shared with two others the 1975 Nobel Prize in Physics for his research on motion in atomic nuclei.

Question 3.
Make the s, p and d – orbital models.
Answer:
Material required: 10 iron spokes, 9 round-shaped wooden pieces, and dumbbell-shaped beads having holes along its length.
Procedure:
- Cut the each iron spoke into three pieces In which one of them Is longer than the other two.
- Make a hole at the middle of the wooden piece and insert the long spoke into that hole.
- Fix the other two spokes to the longer spoke horizontally such that the three spokes are perpendicular to each other.
- Now the three spokes represent X, Y and Z – axis.
- Make nine of these types of models, one for s-orbital, three for p-orbitals and five for d-orbitals.
- Fix the dumb-bell-shaped beeds to the Iron spokes as shown in the fig – to make three (Px, Py, P2) P – orbitals.
- Fix the dumb-bell-shaped beads to the iron spokes as shown in the fig. to make five d-orbitaIs. (dXY,
dYZ,dZX,dX2 – y2 and dX2)
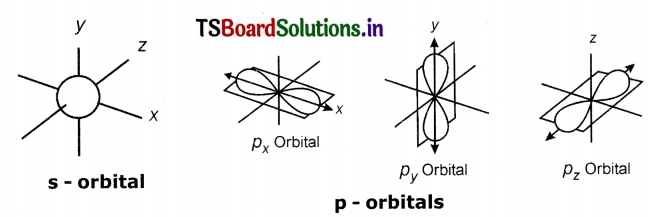
TS 10th Class Physical Science Structure of Atom Intext Questions
Page 106
Question 1.
How do these sub-atomic particles coexist in an electrically neutral atom?
Answer:
Protons and neutrons are present at the centre of the atom called nucleus, and electrons revolve around this nucleus in a circular manner.
As the number of protons and the number of electrons in a neutral atrn1s the same, the +ve charges and -ve charges being equal, they coexist.
Question 2.
Do all atoms have the same sub-atomic particles?
Answer:
Yes, all atoms have same sub-atomic particles but differ in number.
Question 3.
Why is an atom of one element different from the atoms of other elements?
Answer:
Because they have different numbers of sub-atomic particles.
Question 4.
How are the electrons distributed In the space of an atom?
Answer:
Electrons are distributed around the nucleus In subshells of orbits.
Page 107
Question 5.
How many colour sare there in a rainbow?
Answer:
There are seven colours namely violet, Indigo, blue, green, yellow, orange and red (VIBGYOR) in a rainbow.
Question 6.
How do the vibrating electric and magnetic fields around the charge become a wave that travels through space?
Answer:
A vibrating electric charge creates a change In the electric field. The changing electric field creates a changing magnetic field. This process continues, with both the created fields being perpendicular to each other and at right angles to the direction of propagation of the wave.
Question 7.
What are the characteristics of electromagnetic waves?
Answer:
The electromagnetic wave is characterized by wavelength ( λ), frequency (v), velocity (c) and amplitude.
![]()
Page 108
Question 8.
Can we apply C=vλ equation to a sound wave?
Answer:
Yes. It is a universal relationship and applies to all waves. As the frequency increases, the wavelength becomes smaller.
Question 9.
Are there any other wavelengths of light other than visible spectrum?
Answer:
Yes. Cosmic Rays, Gamma Rays, X-rays, U.V. Ray5, I.R. Rays, Micro Waves, Radio Waves are other waves besides visible spectrum.
Page 109
Question 10.
What happens when you heat an iron rod on a flame? Do you find any change In colour on heating an Iron rod?
Answer:
When we heat an Iron rod, some of the heat energy is emitted as light. First It turns red (lower energy corresponding to higher wavelength) and as the temperature rises It glows in orange, yellow, blue (higher energy and of lower wavelength) or even white (all visible wavelengths) colour If the temperature Is high enough.
Question 11.
Do you observe any other colour at the same time when one colour is emitted?
Answer:
When the temperature Is high enough, other colours will also be emitted, but due to higher Intensity of one particular emitted colour (e.g., red), others cannot be observed.
Question 12.
Do you enjoy Deepavall fireworks?
Answer:
Yes, I enjoy because variety of colours are emitted from fireworks.
Question 13.
How do these colours come from fireworks?
Answer:
Inside each handmade crackers, there are small packets filled with special chemicals, mainly metal salts and metal oxides, which react to produce an array of colours. When heated, the atoms of each element in the mixture absorbs energy, causing its electrons to rearrange from their lowest energy state to a higher ‘excited” state. As the electrons plummet back down to their lower energy state, the excess energy gets emitted as light. Each element releases a different amount of energy, and this energy is what determines the colour or wavelength of the light that is emitted.
Page 110
Take a pinch of cupric chloride in a watch glass and make a paste with concentrated hydrochloric acid. Take this paste on a platinum loop and introduce it into a non-luminous flame.
Question 14.
Do you observe yellow light In street lamps?
Answer:
Yes, Sodium vapours produce yellow light in street lamps.
Question 15.
Why do different elements emit different flame colours when heated by the same non-luminous flame?
Answer:
Each element emits Its own characteristic colour when they come back from excited state to ground state. These colours correspond to certain discrete wavelengths of light.
Question 16.
What does a line spectrum tell us about the structure of an atom?
Answer:
We can identify the atom as to what element It belongs. Each element gives a unique light spectrum. That is how spectroscopy works for identifying the elements that make up a certain substance.
Question 17.
What happens when an electron gains energy?
Answer:
The electron moves to a higher energy level that is, the excited state.
Page 111
Question 18.
Does the electron retain the energy forever?
Answer:
The electron loses the energy and comes back to its ground state. The energy emitted by the electron is seen in the form of electromagnetic energy and when the wavelength is in the visible region it is visible as an emission line.
![]()
Question 19.
Did Bohr’s model account for splitting of line spectra of a hydrogen atom Intofiner lines?
Answer:
Bohr’s model failed to account for splitting of line spectra.
Question 20.
Why is the electron in an atom restricted to revolve around the nucleus at certain fixed distances?
Answer:
Because the angular momentum of an electron in an orbit is quantized.
Page 112
Question 21.
Do the electrons follow defined paths around the nudeus?
Answer:
If the electron revolves around the nucleus in definite paths or orbits, the exact position of the electron at various times will be known.
Question 22.
What is the velocity of the electron?
Answer:
3 x 108 m/s
Question 23.
Is It possible to find the exact position of the electron?
Answer:
We can find the probable position of the electron alone but not position and momentum simultaneously.
Question 24.
What do we call the region of space where the electron might be, at a given time?
Answer:
The region or space around the nucleus where the probability of finding the electron Is maximum Is called an atomic orbital.
Page 113
Question 25.
What Information do the quantum numbers provide?
Answer:
The quantum numbers describe the space around the nucleus where the electrons can be found and also their energies. In fact, they give us the address of an electron.
Question 26.
What does each quantum number signify?
Answer:
- The principal quantum number is related to the size and energy of the main shell.
- The azimuthal quantum number gives the shape of a particular sub-shell in the space around the nucleus.
- The magnetic quantum number describes the orientation of the orbital in space relative to the other orbitals in the atom.
- The spin quantum number refers to the two possible orientations of the spin of an electron, one clockwise and the other anticlockwise spin. These are represented by +1/2 and -1/2. If both are positive values, then the spins are parallel otherwise the spins are non-parallel.
Page 114
Question 27.
What is the maximum value of ‘I’ for n=4?
Answer:
Three (Recall, l = n-1)
Question 28.
How many values can ‘I’ have for n=4?
Answer:
9 (2l+1=2×4+1=9)
Question 29.
Do these three p-orbitals have the same energy?
Answer:
The three p-orbitals have same energy and hence these are called degenerate orbitals.
![]()
Page 116
Question 30.
How do electrons In an atom occupy shells, sub-shells and orbitals?
Answer:
Electrons occupy shells, sub-shells and orbitals according to Hund’s rule, Pauli’s rule and Aufbau’s principle.
Question 31.
Helium (Z=2) atom has two electrons. How are these two electrons arranged?
Answer:
Helium atom has two electrons. The first electron occupies ‘is’ orbital. The second electron joins the first In the 1s-orbital, and so the electron configuration of the ground state of He’ is 1s2. According to Pauli Exclusion Principle, no two electrons of the same atom can have all four quantum numbers the same.
If n, l, and ml, are same for two electrons then ms, must be different. In the helium atom the spins must be opposite. Electrons with paired spins are denoted by ‘↓↑’. One electron has m = + 1/2, the other has ms = -1/2. They have non-parallel spins.
Page 117
Question 32.
What are the spins of these two electrons?
Answer:
According to Paull’s Exclusion Principle, no two electrons of the same atom can have all four quantum numbers the same. If n, l, and m are same for two electrons then m must be different. In the helium atom, the spins must be paired. Electrons with paired spins are denoted by ‘↓↑’ one electron has ms=+½, th other has ms= -½. They have anti-parallel spins.
Question 33.
How many electrons can occupy an orbital?
Answer:
An orbital can hold only two electrons and they must have opposite spins.
Page 118
Question 34.
For carbon (C) atom (Z=6), where does the 6th electron go?
Answer:
In carbon, 6th electron enters into Py orbital but not Px orbital.
Question 35.
Whether the electron pairs up In the same p-orbItal or will it go to the next p-orbital?
Answer:
In carbon 6th electron enters into Py orbital but not Px orbital because according to Hunds rule electron pairing does not take place until each degenerate orbital is filled with one electron each.
TS 10th Class Physical Science Structure of Atom Activities
Activity 1
Question 1.
Explain the wave nature of light.
Answer:
- Light is an electromagnetic wave.
- Electromagnetic waves are produced when an electric field and magnetic field are perpendicular to each other.
- This vibrating electric charge creates a change in the electric field. The change in electric field creates a change In magnetic field.
- This process continues, with both the created fields being perpendicular to each other and at right angles to the direction of propagation of the wave.
- The electromagnetic wave is produced.
![]()
Activity 2
Question 2.
Write an activity which shows metals produce different colours In flame.
Answer:
(A)
- Take a pinch of cupric chloride in a watch glass and make a paste with concentrated hydrochloric acid.
- Take this paste on a platinum loop and introduce it into a non-luminous flame.
- Cuprlc chloride produces a green colour flame.
(B)
- Take a pinch of strontium chloride in a watch glass and make a paste with concentrated hydrochloric acid.
- Take this paste on a platinum loop and Introduce It into a non-luminous flame.
- Strontium chloride produces a crimson-red flame.
Activity 3
Question 3.
Complete the electronic configuration of the following elements.
Answer: