Telangana SCERT TS 10th Class Physical Science Study Material Pdf 12th Lesson Carbon and its Compounds Textbook Questions and Answers.
TS 10th Class Physical Science 12th Lesson Carbon and its Compounds
Improve Your Learning
I. Reflections on concepts
Question 1.
What are the general molecular formulae of alkanes, alkenes and alkynes?
Answer:
Alkanes – CnH2n+2
Alkenes – CnH2n
Alkynes – CnH2n-2
Question 2.
Name the product other than water formed on burning of ethanal in air.
Answer:
C2H5OH + 3O2 → CO2 + 3H2O + Energy
Carbon dioxide is another product.
Question 3.
Name the simplest ketone and write Its molecular formula.
Answer:
The simplest ketone is Acetone (Propanone) Its molecular formula is CH3COCH3 (or) C3H6O
Question 4.
Name the compound formed by heating ethanol at 443k wIth excess of conc. H2SO4.
Answer:
Ethanol reacts with conc. H2SO4 at about 443K to give ethene, The reaction is as follows
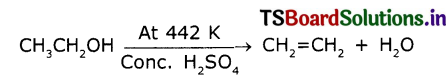
It Is a dehydration reaction. H2SO4 in a dehydrating agent and removes H2O.
Question 5.
Name the product obtained when ethanol is oxidized by either chronic anhydride or alkaline potassium permanganate.
Answer:
Ethyl alcohol (Ethanol) undergoes oxidation to form the product acetaldehyde and finally acetic acid. The reaction Is as follows :
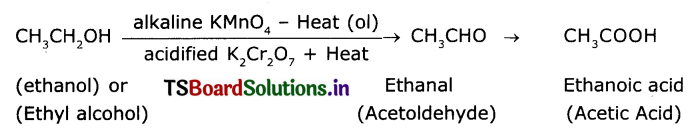
Question 6.
What are homologous series of carbon compounds? Mention any two characteristics of homologous series.
Answer:
Compounds having same functional group are called homologous series. Eq: Alkanes, Alkenes, Alkynes, Halo Alkanes.
Characteristics of homologous series
- They have general formula
Eq: General formula of alkanes CnH2n+2
General formula of alkynes: CnH2n-2 etc. - Successive compounds in the series possess a difference of (-CH2) unit.
- They possess similar chemical properties due to same functional group.
- They show a regular gradation In their physical properties.
![]()
Question 7.
Why does carbon form compounds mainly by covalent bonding’?
Answer:
- Carbon belongs to 14th group or IV A group in the modem periodic table.
- The electronic configuration of carbon is 1s22S22p2. ‘To get octet configuration in its outer shell It has to gain four more electrons to form C4-. Its nucleus has only 6 protons. Therefore it would be difficult for a nucleus with 6 protons to hold 10 electrons. Hence, carbon cannot form C4- ions so easily.
- If carbon loses 4 electrons from the outer shell, it has to form C4+’ ions. This require huge amount of energy which is not available normally, Therefore C4+ formation also is remote possibility.
- Carbon has to satisfy its tetravalency by sharing electrons with other atoms. It has to form ‘4 covalent bonds either with Its own atoms or atoms of other elements.
Question 8.
Explain how sodium ethoxide is obtained from ethanol. Give chemical equations.
Answer:
Ethanol reacts with sodium to liberate hydrogen and forms sodium ethoxide. The chemical equation is
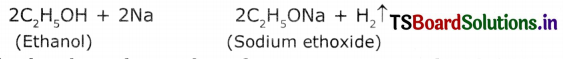
Question 9.
Explain the cleansing action of soap.
Answer:
1. Soaps and detergents make oil and dirt present on the cloth come out into water, thereby making the cloth clean.
2. Soap has one polar end 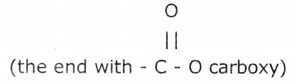 and one non-polar end (the end with the hydrocarbon chain) as shown here,
and one non-polar end (the end with the hydrocarbon chain) as shown here,
3. The polar end Is hydrophilic in nature and this end is attracted towards water.
4. The non-polar end is hydrophobic, in nature and it is attracted towards grease or oil on the cloth, but not attracted towards water.
5. When soap is dissolved in water, its hydrophobic ends attach themselves to dirt and remove It from cloth, as shown sequentially in the figure.
6. The hydrophobic end of the soap molecules move towards the dirt or grease particle.
7. The hydrophobic ends attached to the dirt particle and try to pull it out.
8. The molecule of soap surround the dirt particles at the centre of the cluster and form a spherical structure called micelle.
9. These micelles remain suspended in water-like particles in a colloidal solution.
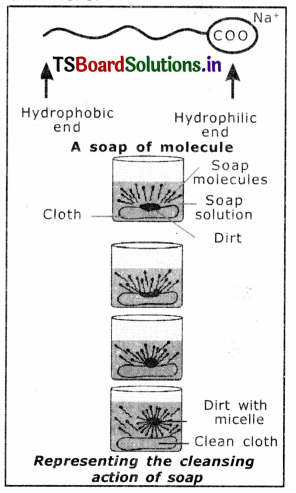
10. The various miscelles present in water do not come together to form a precipitate as each micelle repels the other because of the ion-ion repulsion.
11. Thus, the dirt particles remain; trapped in micelles and are easily rinsed away with water. Hence, soap micelles remove dirt by dissolving in water.
Question 10.
Distinguish between esterification and saponification reactions of organic compounds.
Answer:
| Esterification | Saponification |
| 1) The formation of ester is known as esterification reaction. | 1) The formation of soap is known as saponification reaction. |
| 2) Alcohol reacts with carboxylic acids to produce esters. | 2) Higher fatty acids reacts with bases to form soaps. |
| 3) Water is the by-product in esterification reaction. Eg: Ethyl Acetate (CH3COOC2H5) |
3) Glycerol is the by-product in saponification reaction Eg: Sodium stearate. (C17H35COONa) |
Question 11.
What happens when a small piece of sodium Is dropped Into ethanol?
Answer:
When a small piece of sodium is dropped into ethanol, it shows brisk effervescence and liberates hydrogen gas and forms sodium ethoxide.

Question 12.
Draw the electronic dot structure of ethane molecule (C2H6)
Answer:

Question 13.
Name the simplest hydrocarbon?
Answer:
Methane (CH4) is the simplest hydrocarbon.
Question 14.
Name the carboxylic acid used as a preservative.
Answer:
Acetic acid – CH3COOH (IUPAC Name Ethanoic Acid)
![]()
Question 15.
A mixture of oxygen and ethyne Is burnt for welding; can you tell why a mixture of ethyne and air Is not used?
Answer:
- Air consists many gases like N2, CO2, etc., ¡n addition to oxygen.
- Production of flame for welding is a combustion reaction, which is generally oxidation reaction.
- If air is used it leaves a soot flame. Hence, air is not mixed with ethyne. Only oxygen is mixed with ethyne and used for welding.
Question 16.
What do we call the self linkIng property of carbon?
Answer:
The self-linking property of carbon is called catenation. If any element forms bonds. between its own atoms to give big molecules, we call that property as catenation property
Question 17.
Give an example for esterification reaction.
Answer:
The reaction between carboxylic acid and an alcohol in the presence of Conc.H2SO4 to form a fruity-odoured substance, ester with the functional

Eg Preparation of ethyl acetate from ethanoic acid and ethanol will be as follows:

Question 18.
Write the chemical equation representing the reaction of preparation of ethanol from ethane.
Answer:
- Ethane in the absence of air on heating forms ethanol
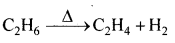
- Ethanol is prepared on large scale from ethene by addition of water vapour to it in the presence of catalysts like P2O5, Tungsten oxide at high pressure a temperature.

Question 19.
Give the names of functional groups (i) -CHO (ii) -C=O.
Answer:
(i) -CHO aldehyde functional group.
(ii) -C=O Ketone functional group.
Question 20.
Explain the structure of graphite In term of bonding and give one property based on this structure.
Answer:
1. Graphite forms a two-dimensional layer structure with C – C bonds within the layers. There are relatively weak interactions between the layers.
2. In a layer structure, the carbon atoms are in a trigonal planar environment. This is consistent with each carbon atom in sp2 hybridization.
3. Interactions between sp2 orbitals leads to the formation of C – C bonds.
4. Each carbon atom is with one unhybridised ‘p’ orbital.
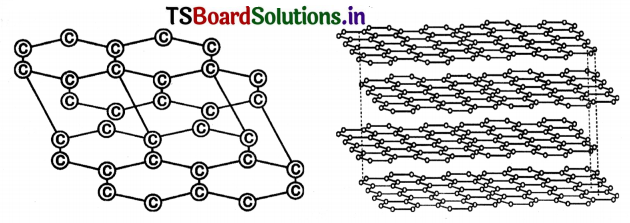
The arrangement of carbon atoms In graphite layers.
5. The unhybridised ‘p’ orbitals interact to form a ‘π’ system that is delocalised over the whole layer.
6. The interactions (or) London dispersion forces between the layers which are separated by a distance of 3.35 Å are weakened by the presence of water. molecules so that It is easy to cleave graphite.
7. For this reason graphite is used as lubricant and as the ‘lead’ in pencils.
Question 21.
Name the acid present in vinegar.
Answer:
- The acid present in vinegar is Ethanoic acid or acetic acid (CH3COOH).
- 5-8% solution of acetic acid in water Is called vinegar.
Question 22.
How do you appreciate the role of esters In everyday lite?
Answer:
Role of esters in everyday life:
- Many esters have pleasant odours, so they are used In perfumes, air refreshers and flavourings, among other things.
- Esters are used to give artificial flavouring for sweets, ice creams and soft drinks. In industries, esters are being used as solvents In the manufacture of, cellulose; varnishes and paints, as solvents in pharmaceutical industries and as softeners or plasticizers in plastic and moulding Industries.
Application Of Concepts
Question 1.
Explain with the help of a chemical equation, how an addition reaction is used In vegetable ghee industry.
Answer:
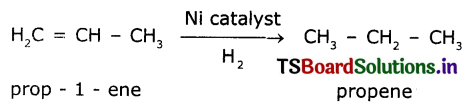
The reaction in which a reagent adds completely on a substance without the removal of small molecules are called addition reaction. Addition of hydrogen in the presence of nickel catalyst to vegetable oil gives ghee. Vegetable oil is unsaturated compound where as ghee is a saturated compound.

Question 2.
a. What are the various possible structural formulae of a compound having molecular formula C3H6O?
b. Give the IUPAC names of the above possible compounds and write their structures.
c. What is the similarity in these compounds?
Answer:
(a) C3H6O
CH3CH2CHO – Ethanal
CH3COCH3 – Propanone
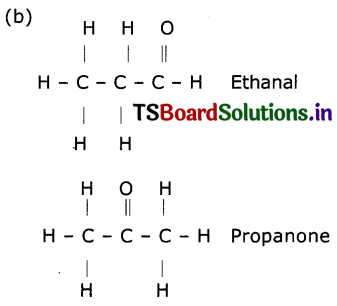
(c) (i) Both ethanol and propanone contain one carbonyl functional group

(ii) Both compounds have same molecular formula,
(iii) Both compounds have the 2sp3 hybridised carbons and one ‘sp2’ hybridised carbon atom.
Question 3.
Allotropy is a property shown by which class of substances: elements, compounds or mixtures? Explain allotropy with suitable examples.
Answer:
Allotropy can be shown by elements.
Allotropy: The property of an element to exist in two or more physical forms having more or less chemical properties but different physical properties is called ‘allotropy’.
Carbon has many allotropes. They are classified into two types.
1) Amorphous forms
2) Crystalline forms
- Amorphous forms: Different amorphous forms of carbon are coal, coke, wood charcoal, animal charcoal, lamp black, etc.
- Crystallise forms: Different crystalline forms of carbon are diamond, graphite, buckminsterfullerene, nanotubes etc.
Question 4.
Two carbon compounds A and B have molecular formulae C3H8 and C3H6 respectively. Which one of the two is most likely to undergo addition reactions? Justify your answer.
Answer:
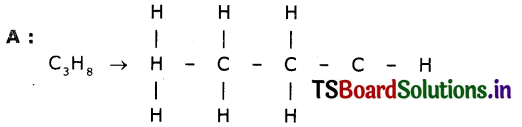
It is a saturated hydrocarbon. It shows substitution reaction.
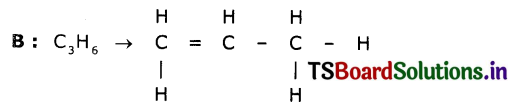
This is an unsaturated hydrocarbon. Hence it shows addition to become saturated. During the reactions, addition of reagent takes place at the double-bonded carbon atoms.
![]()
Question 5.
1 ml of glacial acetic acid and 1 ml of ethanol are mixed together in a test tube. Few drops of concentrated sulphuric acid is added to the mixture and warmed ¡n a water bath for 5 min. Answer the following:
(a) Name the resultant compound formed.
(b) Represent the above change by a chemical equation?
(c) What name is given to such a reaction?
(d) What are the special characteristics of the compound formed?
Answer:
(a) Ethyl acetate (CH3COOC2H5) an ester.
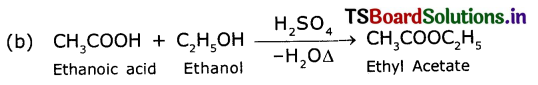
(c) Esterification reaction
(d) The formed compound when poured into water, we observe a sweet fruit odour.
Question 6.
Give the IUPAC name of the following compounds. If more than one compound is possible name at least two of them.
(i) An aldehyde derived from ethane.
(ii) A ketone derived from butane.
(iii) A chloride derived from propane.
(iv) An alcohol derived from pentane.
Answer:
(i) An aldehyde derived from ethane is “Ethanal” (CH3CHO) (or) 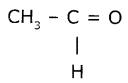
(ii) Ketone derived from butane s Butanone (Or) Butane-2- one.
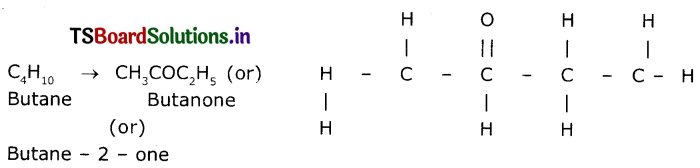
(iii) Chloride derived from propane is
(a) Propyl chloride
CH3 – CH2 – CH2Cl
(b) 2 – Chloro – Propane

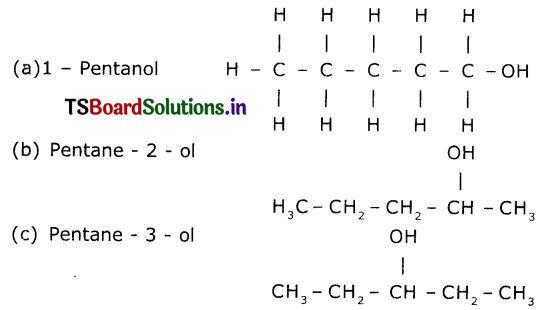
(iv) Alcohol derived from pentane
Question 7.
Write the IUPAC name of the next homologous of CH2OHCH2CH3.
Answer:
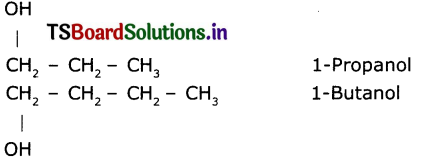
Question 8.
How do you condemn the use of alcohol as a social practice?
Answer:
- Consumption of alcohol ¡n the form of beverages is harmful to health.
- It causes severe damage to blood circulation system.
- Addiction to alcohol drinking leads to heart diseases and damages the liver.
- It also causes ulcers in small intestines due to increased acidity and damages the digestive system.
- Alcohol which is consumed in raw form under the names liquor, gudumba is more harmful to health due to adulteration.
- Alcohol mixed with pyridine is called denatured spirit. Consumption of denatured spirit causes blindness and death.
- Hence use of alcohol is a social evil which harms the society.
Question 9.
An organic compound with molecular formula C2H4O2 produces brisk effervescence on addition of sodium carbonate/bicarbonate.
Answer the following:
(a) Identify the organic compound.
(b) Write the chemical equation for the above reaction.
(C) Name the gas evolved.
(d) How will you test the gas evolved?
(e) List two important uses of the above compound.
Answer:
a) CH3COOH (EtharioiC acid)
b) 2CH3COOH + Na2CO3 → 2CH3COONa + H2 + CO2
CH3COOH + NaHCO3 → CH3COONa + CO2 + H2O
C) Carbon dioxide (CO2)
d) When the evolved gas is passed into lime water, lime water turns milky white. Basing on the observation we conclude that the evolved gas is carbon dioxide.
e) Ethanoic acid Es used in
a) for preservation of pickles
b) for preparation of dyes, drugs
C) for solvent in industry
d) for curing meat, fish.
Multiple choice questions
Question 1.
Which of the following solution of acetic acid in water can be used as preservative? [ ]
(a) 5-10%
(b) 10-15%
(c) 15-20%
(d) 100%
Answer:
(a) 5-10%
Question 2.
The suffix used for naming an aldehyde is [ ]
(a) -0l
(b) -al
(c) -one
(d) -ene
Answer:
(b) -al
Question 3.
Acetic acid, when dissolved in water, it dissociates into ions reversibly because it is a:[ ]
(a) Weak acid
(b) strong acid
(c) weak base
(d) strong base
Answer:
(a) Weak acid
![]()
Question 4.
Which one of the following hydrocarbons can show isomerism? [ ]
(a) C2H4
(b) C2H6
(c) C3H8
(d) C4H10
Answer:
(d) C4H10
Question 5.
Combustion of hydrocarbon is generally accompanied by the evolution of [ ]
(a) Heat
(b) Light
(c) both heat and light
(d) Electric current.
Answer:
(c) both heat and light
Question 6.
2m1 of ethanoic acid was taken in each of the three test tubes A, B and C’ and 2m1. 4ml and 8 ml of water was added to them, respectively. A clear solution is obtained in: [ ]
(a) Test tube A only
(b) Test tubes A & B only.
(c) Test tubes B and C only
(d) All the test tubes.
Answer:
(d) All the test tubes.
Question 7.
If 2 ml of acetic acid was added slowly in drops to 5m1 of water then we will notice [ ]
(a) The acid forms a separate layer on the top of water.
(b) Water forms a separate layer on the top of the acid.
(c) Formation of a clear and homogenous solution.
(d) Formation of a pink and clear solution.
Answer:
(c) Formation of a clear and homogenous solution.
Question 8.
A few drops of ethanoic acid were added to solid sodium carbonate. The possible results of the reactions are: [ ]
(a) A hissing sound was evolved
(b) Brown fumes evolved.
(c) Brisk effervescence occurred.
(d) A pungent-smelling gas evolved.
Answer:
(c) Brisk effervescence occurred.
Question 9.
When acetic acid reacts with ethyl alcohol, we add cone. H2SO4, which acts as and the process is called [ ]
(a) Oxidizing agent, saponification
(b) Dehydrating agent, estenfication
(c) Reducing agent, Esterification
(d) Acid & esterification
Answer:
(b) Dehydrating agent, estenfication
Suggested Experiments
Question 1.
Suggest a test to find the hardness of water and explain the procedure.
Answer:
Hardness of water can be tested with the help of a good quality soap.
Procedure
- Take 50ml of water from different sources i.e., tap water, well water, lake water, pond water, river water, etc., in different test tubes and lable them as A, B, C, D etc.
- Add 1gm of good-quality soap to each test tube.
- Close each test tube with rubber corks.
- Shake test tube A for 15 seconds and keep it undisturbed for 30 seconds. Measure the height of the foam formed. Note the height of foam in your notebook.
- Repeat the process for each test tube and record your observation in your notebook.
- The water which gives less foam is considered as hard water.
Question 2.
Suggest a chemical test to distinguish between ethanol and ethanoic acid and explain the procedure.
Answer:
- Take ethanol and ethanoic acid in two different test tubes.
- Add nearly 1gm of sodium bicarbonate (NaHCO3) to each test tube.
- Lots and lots of bubbles and foam will be observed from the test tube containing ethanoic acid. This is due to release of CO2.
NaHCO3 + CH3COOH → CH3COONa + H2O + CO2 - Ethanol will not react with sodium bicarbonate and thus we won’t observe any change in the test tube containing ethanol. Thus we can seperate ethanol from ethanoic acid.
Question 3.
An organic compound ‘X’ with a molecular formula C2H6O undergoes oxidation with alkaline KMnO4 and forms the compound ‘Y’, that has molecular formula C2H4O2.
(a) Identify “X”and “Y”.
Answer:
X : C2H6O is Ethanol
Y: C2H4O2 is Ethanoic acid
Ethyl alcohol undergoes oxidation to form the product Acetaldehyde and finally Acetic acid.

This CH3COOH is used as preservative for pickles.
(b) Write your observation regarding the product when compound ‘X’is made to react with compound “Y” which ¡s used as a preservative for pickles.
Answer:
When x (ethanol) is reacted with y (ethanoic acid) in the presence of concentrated H2S04 to form fruity odour substance called ester (ethyl acetate)

Here CH3COOH is used as preservative for pickles.
![]()
Suggested Projects
Question 1.
Prepare models of methane, ethane, ethene and ethyne molecules using clay balls and match sticks.
Answer:
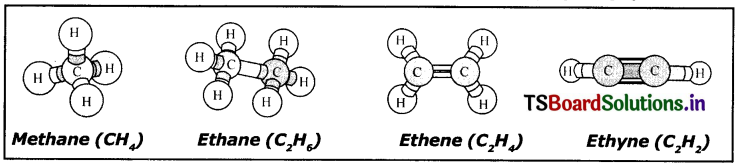
in these models Black Ball is Carbon atom White Ball is Hydrogen atom.
Question 2.
Collect information about artificial ripening of fruits by ethylene.
Answer:
Chemistry of ripening:
- During ripening, the starch in the fruit breaks down to form sugar. The fruit skin changes its colour.
- The ripening of fruit depends on the season. The plant can detect the changes in season. They produce ethylene (C2H4) which spreads across the plant.
- When ethylene reaches the fruits, it sends a signal to ail the cells in the fruit to make enzymes which break starch into sugar.
- The cells in the skin start making pigments, which give the fruit its colour.
Artificial ripening:
- Raw fruits are kept in hay-lined wooden boxes called crates. These crates are stacked on shelves and a wood fire s lit below them. The smoke contains ethylene and acetylene gases, which induce ripening.
- Fruits are placed in a room into which ethylene gas or acetylene gas is introduced.
- In another method calcium carbide (CaC2) is applied over fruits. It reacts with moisture to form acetylene, which induces ripening.
TS 10th Class Physical Science Carbon and its Compounds Intext Questions
Page 253
Question 1.
Can carbon get helium configuration by losing four electrons from the outer shell?
Answer:
No. if carbon loses four electrons from the outer shell, it has to form C+4 ions. This requires huge amount of energy which is not available normally.
Page 254
Question 2.
How do carbon atoms form bonds in so many ways
Answer:
Electronic configuration of carbon (ground state) is 1S2 2S2 2P2 (or) 1S2 2S2 2Px1 2Py1 2Pz1
![]()
Electronic configuration of carbon (excited state):
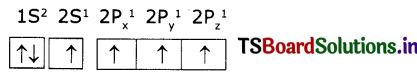
In excited state of carbon atom one ‘2s’ electron Is promoted to ‘2Pz’ orbital. Each carbon In excited state has four unpaired electrons and forms four covalent bonds as shown below.
Question 3.
Explain the four unpaired electrons in carbon atom through excited state
Answer:
Electronic configuration of carbon (ground state)
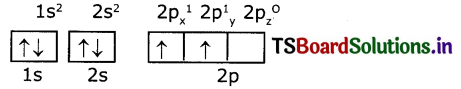
Electronic configuration of carbon (excited state)
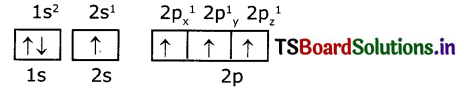
So, in 2s,2p orbitals there are four unpaired electrons In each of 2s, 2px, 2py, and 2pz orbitals.
Page 255
Question 4.
Where this energy to excited electron comes from?
Answer:
The energy required for excitation is taken up from bond energies, which are liberated energies and four bonds are formed between carbon atoms and other atoms.
Question 5.
In methane (CH4) molecule, all four carbon-hydrogen bonds are identical and bond angle ![]() is 109° 28’. How can we explain this?
is 109° 28’. How can we explain this?
Answer:
In methane (CH4), in excited state, carbon atom has three unpaired electrons in p-orbitals, and one electron in s-orbital. So all four C-4 bonds are identical.
Question 6.
How these energetically unequal valence electrons form four equivalent covalent bonds in methane molecules?
Answer:
- The energy difference between the 2s and 2p orbitals is very small.
- When carbon atom is ready to form bonds it gets a small amount of energy from bond energies and gets excited and is promoted from the ‘2s’ to the empty 2pz to give four unpaired electrons.
- These four unpaired electrons form four equivalent covalent bonds in methane.
Page 259
Question 7.
What are bond angles of ![]() in CH4, C2H4 and CH3 molecules?
in CH4, C2H4 and CH3 molecules?
Answer:
The bond angles:
In CH4 is 109°28′
In C2 H4 is 120°
In C2H2 Is l80°
Page 261
Question 8.
How do you understand the marking (writings) of a pencil on a paper?
Answer:
When we write with a pencil, the interlayer attractions break down and leave graphite layers on the paper. These pencil marks are easy to remove from paper with an eraser because the layers do not bind strongly to the paper.
![]()
Page 264
Question 9.
AllottIng completely one special branch in chemistry to compounds of only one element. Is It justified when there are so many elements and their compounds but not with any special branches?
Answer:
- We know that all molecules that make life possible, namely, carbohydrates, proteins, nucleic acids, lipids, harmones and vitamins contain carbon.
- The chemical reactions that take place in living systems are of carbon corn pounds.
- Food that we eat, various medicines we use cotton and silk fibres, synthetic fibres, plastics, synthetic rubber are also compounds of carbon.
- So, Carbon Is a unique element with the largest number of compounds.
- So allotting completely one special branch in chemistry to carbon is justified.
Page 265
Question 10.
What are hydrocarbons?
Answer:
The compounds containing only carbon and hydrogen in their molecules are called ‘Hydrocarbons’.
Question 11.
Do all the organic compounds have equal number of ‘C’ and ‘H’ atoms?
Answer:
No. Different organic compounds have different number of ‘C’ and ‘H’ atoms.
Page 266
Question 12.
Can carbon form bonds with the atoms of other elements?
Answer:
It Is observed that carbon atoms form compounds not only with hydrogen atoms but also with atoms of other elements like oxygen, sulphur, nitrogen, phosphorus, halogens etc. These atoms are called heteroatoms and the compounds are formed with particular functional groups.
Page 269
Question 13.
How about their structures? Are they same?
Answer:
The structures are not the same. They are different.
Question 14.
How many carbon and hydrogen atoms are there In (a) and (b) structures?
Answer:
In structure (a) there are 4 carbon atoms and 10 hydrogen atoms.
In structure (b) also there are 4 carbon atoms and 10 hydrogen atoms.
Question 15.
Write the condensed molecular formulae for (a) and (b). Do they have same molecular formulae?
Answer:
The condensed molecular formula for (a) is C4H10.
The condensed molecular formula for (b) is C4H10.
Both (a) and (b) have the same condensed molecular formula. (Isomers)
Page 280
Question 16.
Why do sometimes cooking vessels get blackened on a gas or kerosene stove?
Answer:
Because of Inlets of air getting closed, the fuel gases do not completely undergo combustion. Hence, it forms a sooty carbon form (black in colour) which gets coated over the vessels.
Page 283
Question 17.
Do you know how the police detect whether suspected drivers have consumed alcohol or not?
Answer:
- The police officer asks the suspected driver to blow air into a plastic bag through a mouthpiece of the detecting instrument which contains orange-coloured crystals of potassium dichromate (K2Cr2O7).
- As K2Cr2O7 is a good oxidising agent ethanol which is in the driver’s breath changes to ethanoic add. The orange colour changes to green and the police can detect that the driver has consumed alcohol.
Page 284
Question 18.
What are esters?
Answer:
- The reaction between carboxylic acid and an alcohol ‘n the presence of conc H2SO4 produces a sweet-smelling substance called ester.
- An ester contains the functional group: – C – COOR
Question 19.
What do you notice?
Answer:
We notice that the resulting mixture is a sweet-smelling substance and is known as ethyl acetate, an ester.
Page 285
Question 20.
Do you know what ‘soap’ is?
Answer:
Soap is a sodium or potassium salt of a higher fatty acid like palmitic acid (C15H31COOH), stearic acid (C17H35COOH) Oleic acid (C17H33COOH) etc.
The formula of soap is RCOONa (or) RCOOK
R = C15H31; C17,H35 etc.
Page 286
Question 21.
What isatrue solution?
Answer:
A true solution is that in which the solute particles dispersed In the solvent are less than mm (10-9m) in diameter.
Question 22.
Can you see the oil and water layers separately in both the test tubes.
Answer:
(a) Immediately after: You stop shaking them?
Answer:
No,
(b) Leave the test tubes undisturbed for sometime and observe. Does the oil layer separate out?
Answer:
Yes. After allowing test tubes to settle for some time. the oil layer floats on water layer.
(c) in which test tube does this happen first? Give your observations?
Answer:
The oil layer and water layer separate first in test tube B, with soap water.
Page 287
Question 23.
What is the action of soap particles on the greasy cloth?
Answer:
The soap particles make oil and dirt present on the cloth come out into water, thereby making the cloth clean.
Think And Discuss
Question 1.
Why we are advised not to use animal fats for cooking?
Answer:
Fats are generally solids at room temperature. Animal fats have saturated carbon chains. Hence they require large quantities of heat to break those chains. In this process a lot of fuel is consumed. So, we are advised not to use animal fats for cooking.
Question 2.
Which oil is recommended for cooking? Why?
Answer:
Generally, vegetable oils having long unsaturated carbon chains are recommended for cooking.
Reasons:
- They do not require large quantities of heat to break the carbon chains.
- So we can save cooking fuel.
- Secondly, it does not take long time to cook food which prevents over cooking which is harmful to health.
TS 10th Class Physical Science Carbon and its Compounds Activities
Activity 1
Question 1.
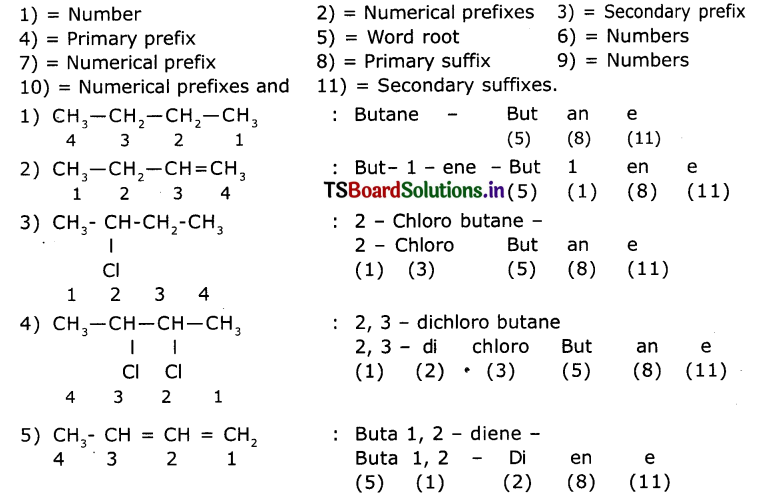
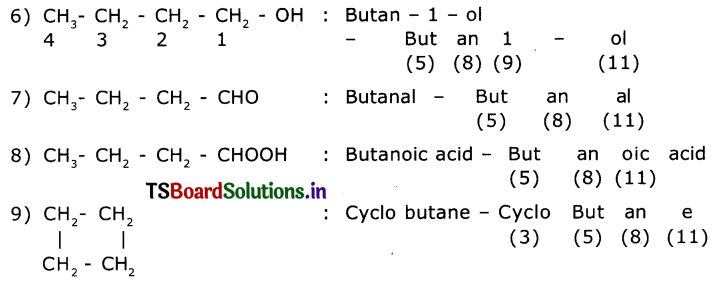
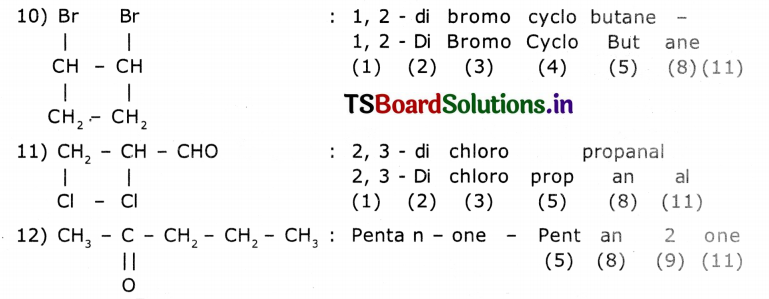
Observe the names of the following compounds. Give reasons in the space provided. (ASS) (4 Marks)
Answer:
COOH > (CH3CO)2O> – COOR > -COX> -CONH2 > -CN > – CHO > C=O > R-OH> -NH
Acid anhydride ester acid halide amide nitrite aldehyde ketones alcohols amines
Divide the given names as per the notations given and identify the parts In the name through the numbers given from (1) to (11) and write them in your notebook.
![]()
Activity 2
Question 1.
Write an activity to show esterification.
Answer:
- Take imI of ethanol and imI of glacial acetic acid along with a few drops of concentrated sulphuric acid in a test tube.
- Warm it In a water bath or a beaker containing water for at least 5 minutes as shown In fig.
- Pour the warm contents into a beaker containing 20-50 ml of water and observe the odour of the resulting mixture.
Observation: The resulting mixture Is a sweet odoured substance. This substance is ester. This reaction is esterification.
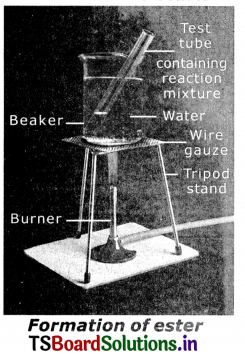
Activity 3
Question 1.
How do you test the cleansing action of soap?
Answer:
1. Take about 10 ml of water in two test tubes.
2. Add a drop of cooking oil to both the test tubes and label them as A and B.
3. Add a few drops of soap solution to test tube B.
4. Now shake both the test tubes vigorously for the same period of time.
5. We cannot observe that the oil and water layers separately in both the test tubes immediately after shaking Is stopped.
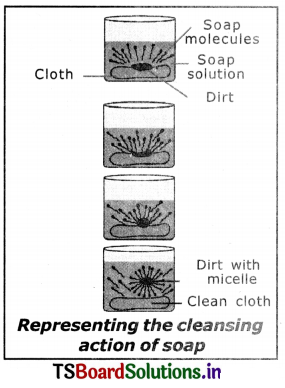
6. Leave the test tubes undisturbed for some time and observe.
7. We observe the separation of oil layer in test tube B first because it contains the soap solution.
8. This gives an idea of cleansing action of soap.