These TS 10th Class Physical Science Chapter Wise Important Questions Chapter 12 Carbon and its Compounds will help the students to improve their time and approach.
TS 10th Class Physical Science Important Questions Chapter 12 Carbon and its Compounds
1 Mark Questions
Question 1.
What is allotropy’’
Answer:
An element exists in two or more different forms due to the difference in their atomic arrangement is called Allotrophy” and the different forms are called allotropes.
Question 2.
Explain briefly about the structure of ‘Diamond’.
Answer:
- In a diamond, each ‘c’ is surrounded by four other ‘c’ atoms.
- These are placed at the four corners of a regular tetrahedron.
- This results in a 3-dimensional network of carbon atoms.
Question 3.
Explain briefly about the structure of “Graphite”.
Answer:
- In graphite, each ‘c’ is surrounded by three other ‘c atoms.
- The ‘c’ atoms are arranged in layers.
- Each layer consists of a 2-dimensional hexagonal network.
Question 4.
“Diamond is an extremely bad conductor of electricity.” Why?
Answer:
- In a diamond, each carbon atom is covalently bonded with four other carbon atoms.
- So, the four outermost electrons of a carbon atom are engaged or trapped in the covalent bonds, having no free electrons makes it a bad conductor of electricity.
Question 5.
What is an alkyl group’
Answer:
If one hydrogen is removed from an alkane, it is called alkyl group.
Eg : CH4 → methane
CH3 → methyl group
Question 6.
What is the bond length in diamond?
Answer:
The C – C bond length In diamond is 1.54 Å.
![]()
Question 7.
What is polymerization?
Answer:
The reaction In which a large number of identical and simple molecules Join together to form a large molecule is called “Polymerization.
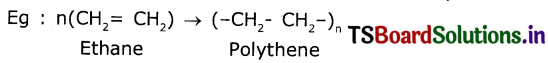
Question 8.
Name some functional groups.
Answer:
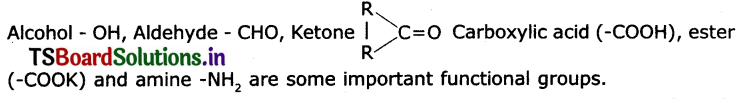
Question 9.
What is the bond angle in diamond?
Answer:
The bond angle in diamond is 109° 28′.
Question 10.
What is the C-C bond length in graphite?
Answer:
The C-C bond length in graphite is 1.42 A.
Question 11.
What is the bond angle in graphite?
Answer:
The bond angle in graphite is 120°.
Question 12.
What is hydrocarbon?
Answer:
Compounds containing only carbon and hydrogen are called hydrocarbons.
Eg: Alkanes (Saturated hydrocarbons)
Alkenes and Alkynes (unsaturated hydrocarbons)
Question 13.
What are saturated hydrocarbons? (or) What are alkanes?
Answer:
The valency of the carbon is four 4, of all the valencies of carbon are satisfied, the resultant hydrocarbons are refer to as saturated hydrocarbons or Alkanes. They general formula is CnH2n+2.
Question 14.
What are unsaturated hydrocarbons?
Answer:
The hydrocarbons containing one or more double bonds or triple bonds between two carbon atoms are called “Unsaturated hydrocarbons.
Eg: C2H6 and C3H6 etc.
![]()
Question 15.
What are alkanes? Write the general formula of alkenes. Give an example for alkenes.
Answer:
Alkenes: Hydrocarbons containing at least one double bond between carbon atoms are called Alkenes (>C=C<)
General formula: CnH2n
Examples : Ethene (C2H4, Propene (C3H6)
Question 16.
What are Alkynes?
Answer:
Alkynes are unsaturated hydrocarbons having at least (CC) triple bonds In these structures. Their general formula is CnH2n-2.
Eg: Acetylene (HC ≅ CH)
Question 17.
What is Homologous series?
Answer:
A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called a homologous series.
Question 18.
Give the names of the functional group.
Answer:
(i) – CHO- Aldehyde

(iii) – OH – Alcohol
(iv) – COOH – Acid (carboxylic acid)
Question 19.
What do you mean by a functional group?
Answer:
The characteristic properties of an organic compound depend mainly on an atom or group of atoms In its molecule known as the ‘functional group’, Functional group is responsible for the behavior of the organic compounds.
Question 20.
What is the difference between two successive homologs?
Answer:
Successive compounds in a homologous series possesses a difference of (CH3) unit and are called homologs.
Question 21.
Write the structure of 3 – bromo – 2 – chloro – 5 0x0 hexanoic acid.
Answer:
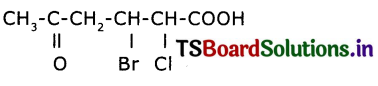
Question 22.
Why graphite is a good conductor of electricity?
Answer:
Graphite Is a good conductor of electricity because of the delocalized π electron system.
Question 23.
What are the uses of fullerenes?
Answer:
Fullerenes are under study for potential medicinal use such as specific antibiotics to target resistant bacteria and even target certain cancer cells such as melanoma.
![]()
Question 24.
What are oxidizing agents?
Answer:
Oxidizing agents or oxidants are substances that oxidize other substances.
Question 25.
Why do we call alkanes as paraffins?
Answer:
Alkanes are saturated hydrocarbons with least reactivity. Therefore they are called paraffins (Parum = little: affine affinity).
Question 26.
Write two uses of Ethanol In day-to-day life.
Answer:
Ethanol is used in
- Preparation of Alcoholic drinks
- Preparing tincture iodine
- Preparing cough syrup and tonics
Question 27.
What is the use of ethanol ¡n motors?
Answer:
If we add 10% ethanol to gasoline it will act as very good motor fuel.
Question 28.
What is a colloidal solution?
Answer:
If the diameter of dispersed phase Is greater than 1 nm but less than 1000 nm in a dispersed medium such a solution Is called colloidal solution.
Question 29.
Other than carbon which elements show catenation?
Answer:
Sulphur, phosphorus, and silicon.
Question 30.
What are the other names given to open-chain hydrocarbons?
Answer:
Open-chain hydrocarbons are also called as aliphatic hydrocarbons or acyclic hydrocarbons.
Question 31.
Expand IUPAC.
Answer:
International Union of Pure and Applied Chemistry.
Question 32.
Name the following compound.
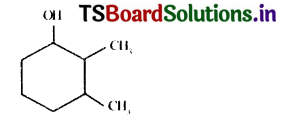
Answer:
2, 3 – dimethyl – cyclo hexane- 1 – ol
Question 33.
How do you appreciate the role of diamonds In space probes?
Answer:
Since it has the ability to filter out harmful radiation, it is used in making protective windows for space probes.
Question 34.
How do you appreciate the role of diamonds in surgery?
Answer:
A sharp-edged diamond is used as a tool to remove cataracts In eye surgery.
Question 35.
How do you appreciate the role of oxygen in combustion process?
Answer:
- When the oxygen supply is insufficient then the fuels burn incompletely Hoducing mainly a yellow flame.
- When the oxygen supply is sufficient then the fuels burn completely producing
a blue flame.
Question 36.
How do you appreciate the role of Ethanol as a fuel?
Answer:
- A material which Is burnt to obtain heat is called a fuel. Since ethanol burns with a clear flame giving a lot of heat, it is used as a fuel.
- Some countries add ethanol to petrol to be used as a fuel in cars. Thus ethanol is used as an additive in petrol.
- Ethanol alone can also be used as a fuel for cars.
Question 37.
How do you appreciate the role of active acid as a preservative?
Answer:
- Dilute acetic acid Is used as a food preservative In the preparation o! pickles and sauces.
- As vinegar, it is also used as an appetizer for dressing food dishes.
Question 38.
How do you detect leakage in the cylinder?
Answer:
- To detect any leakage of gas from the cylinder a strong-smelling substance like ethyl mercaptan (C2H5SH) Is added to the gas.
- Then the leakage can be easily detected by the foul smell of the ethyl mercaptan.
Question 39.
What are nanotubes?
Answer:
Nanotubes are allotropic form of carbon.
![]()
Question 40.
Write the molecular formula of the fourth member of the homologous series of alcohols.
Answer:
CH3– CH2– CH2 – CH2– OH
Question 41.
What is a catalyst?
Answer:
The substance which does not take part In chemical reaction but changes the rate of reaction is called a catalyst.
Question 42.
Why oils are in liquids at room temperature?
Answer:
Oils are unsaturað compounds so they are In liquid state.
Question 43.
Why fats are solids at room temperature?
Answer:
They are saturated compounds so they are in solid state.
Question 44.
Do you know the police detect whether suspected drivers have consumed alcohol or not? Explain.
Answer:
Orange Cr2O72- changes bluish-green Cr3+ during the process Of the oxidation of alcohol. The Ieiqth of the tube that turned into green is the measure of the quantity of alcohol that had been drunk.
Question 45.
What is pica?
Answer:
The negative value of logarithm of dissociation constant of an acid.
Question 46.
Name the gas evolved when acetic acid is react with sodium hydrogen carbonate.
Answer:
The gas liberated is carbon dioxide.
Question 47.
Name the organic add present In vinegar – write Its chemical formula.
Answer:
The acid present In vinegar is acetic acid. its formula is CH3COOH.
Question 48.
What change will you observe If you test soap with litmus papers?
Answer:
Red litmus turns into blue.
![]()
Question 49.
Write the valency of carbon In CH3– CH3, CH2 = CH2, and HC ≅ CH?
Answer:
The valency of carbon in CH3– CH3, is 4.
The valency of Carbon in CH2 = CH2 is 3.
The valency of carbon In HC ≅ C – H is 2.
Question 50.
Out of butter and ground nut oil which Is unsaturated In nature?
Answer:
Ground nut oil is unsaturated In nature.
Question 51.
What are hydrophobic and hydrophilic parts in soap?
Answer:

Question 52.
Name the carboxylic acid used as preservation.
Answer:
Acetic acid used as preservative.
Question 53.
Write two uses of nanotubes.
Answer:
- Nanotubes are used to connect the components of integrated circuits instead of copper.
- Biomolecules are inserted into nanotubes to inject them into a single cell.
2 Marks Questions
Question 1.
Identify the functional groups In the following compound arid write IUPAC names.
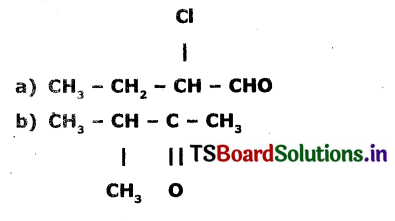
Answer:
a) The functional group present In 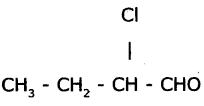 is aldehyde.
is aldehyde.
The IUPAC name of the compound is 2 – Chloro-Butan- 1 – ol.
b) The functional group present in 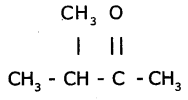 is ketone.
is ketone.
The TUPAC name of the compound is 3- Methyl-2-Butanone.
Question 2.
What are alkenes?
Answer:
Alkenes are unsaturated hydrocarbons having at least one(C=C) double bond in these structures. Alkenes are also called olefins. Their general formula is Cn– H2n Eg: Ethylene (C2H4) and propene (C3-H6) etc.
![]()
Question 3.
Which of the following compounds are unsaturated? Justify your answer.
(a) CH3-CH2-CH3
(b) CH3 – CH = CH2
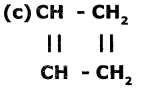
(d) HC ≅ C – CH = CH2

Answer:
a and f are saturated hydrocarbons because they have only single bonds. Remaining are unsaturated hydrocarbons as they have double or triple bonds.
Question 4.
What are hydrophilic and hydrophobic parts In soaps? (AS1)
Answer:
The polar end in soap with carboxy 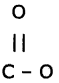 is called hydrophilic end. The non-polar end ¡n soap with hydrocarbon chain is called hydrophobic end.
is called hydrophilic end. The non-polar end ¡n soap with hydrocarbon chain is called hydrophobic end.

Question 5.
What is the order to be followed while naming carbon compounds?
Answer:
The following order is to be followed while naming carbon compounds. Numbers-Numerical Prefixes-Secondary Prefix-Primary Prefix-Word Root-

Question 6.
Explain addition reactions.
Answer:
Unsaturated organic compounds that contain multiple bonds like alkenes and alkynes became saturated by addition of a reagent are called addition reactions. During the reactions addition of the reagent takes place at the double-bonded or triple-bonded carbon atoms.
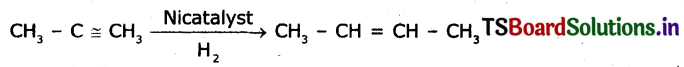
Question 7.
What type of reaction takes place between ethane and chlorine?
Answer:
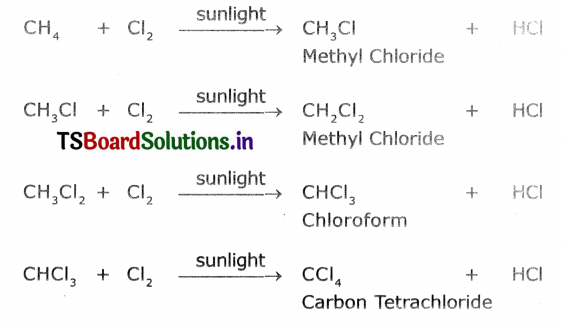
Substitution reaction takes place between ethane and chlorine In the presence of sunlight.
CH4 + Cl2 → CH3Cl + HCl
CH3Cl+ Cl2 → CH2Cl2 + HCl
CH2Cl2 +Cl2 → CHCl3 + HCl
CHCl3 + Cl2 → CCl4 + HCl
![]()
Question 8.
An organic compound X with a molecular formula C2H6O undergoes oxidation with in presence of alkaline KMnO4 to form a compound y. X on heating in presence of con. H2SO4 at 443 k gives Z. Which on reaction with Br2 and decolorizes, It. Identity X, Y, and Z write the reactions involved.
Answer:
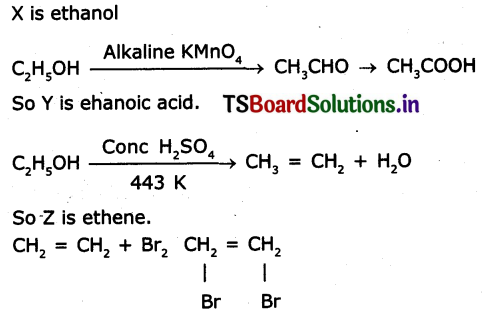
Question 9.
How synthetic detergents are harmful for environment?
Answer:
- Some synthetic detergents resist biodegradation, i.e., they are not decomposed by microorganisms such as bacteria.
- Hence, they cause water pollution In lakes and rivers.
- They tend to persist for a long time, making the water unfit for aquatic life.
Question 10.
Write the reactions of Ethanol with sodium and conc. H2SO4
Answer:
- Ethanol reacts with sodium metal to liberate hydrogen and forms sodium ethanoxide.
2C2H5OH + 2Na → 2C2H5ONa + H2 - Ethanol reacts with conc. H2SO4 at about 170°C to give ettiene.
CH3CH2OH CH2 = CH2 + H2O
Question 11.
Write the reactions of Ettianoic acid with metals, metal hydroxide, metal carbonates, and metal hydrogen carbonates.
Answer:
1. ReactIon of ethanoic acid with metal: Ethanoic add reacts with active metals like Na to liberate hydrogen.
2CH3COOH + 2Na → 2CH3COONa + H2
2. Reaction of ethanoic acid with metal hydroxide: Ettianoic acid reacts with NaOH to form salt and water.
CH3COOH + NaOH → CH3COONa + H2O
3. Reaction of ethanoic acid with metal carbonates and metal hydrogen carbonates: Ethanoic acid reacts with sodium carbonate and sodium hydrogen carbonate and liberates CO2
2CH3COOH + Na2CO3 → 2CH3COONa + H2O + CO2
CH3COOH + NaHCO3 → CH3COONa + H3O + CO2
4 Marks Questions
Question 1.
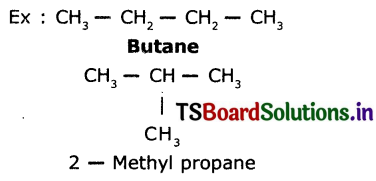
Draw the structures of Isomers of butane.
Answer:
Isomers of butane are n-butane, isobutane and cyclobutane Structures:
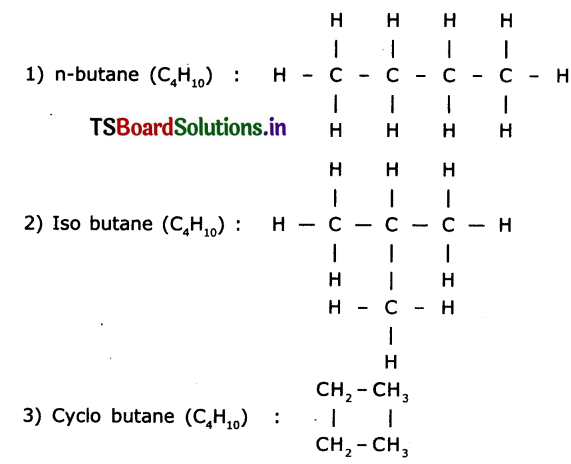
Question 2.
explain the Isomerism and Catenation properties of Carbon.
Answer:
Catenation properties of carbon :
(i) Carbon has ability to form longest chains with its own atoms. This special property of carbon is called catenation.
(ii) Due to catenation property of carbon it can form largest chain containing millions of carbon atoms, branches and acidic compounds.
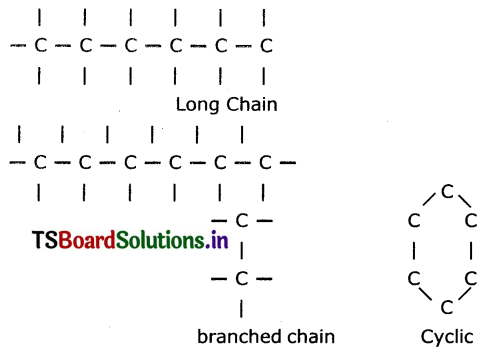
Isomerism of carbon: The phenomenon of possessing some molecular formula but different properties by the compounds is known as isomerism.
The molecular formula of above two molecules is C4H10 but they have different structures.
These two are isomers
By there two special properties of carbon it can make number of compounds.
Question 3.
Draw the structures of the following.
a) Ethanoic acid
b) Propanol
c) Propene
d) Chloro propene
Answer:
Structures
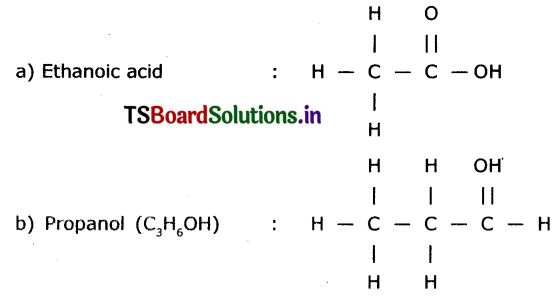
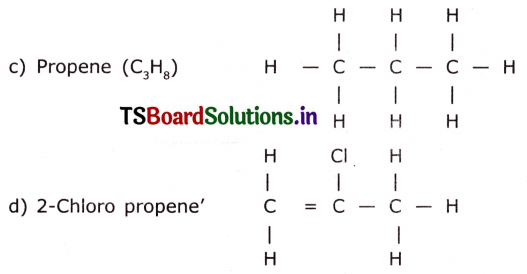
Question 4.
Draw structure of Ethane and electron dot structure of Chlorine.
Answer:
Ethane:
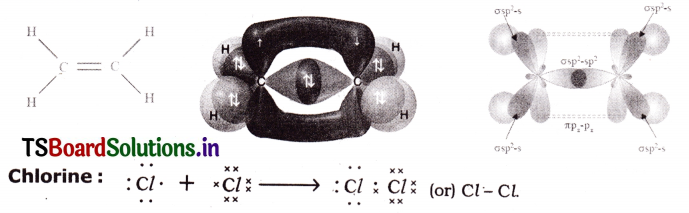
Question 5.
Draw the structures of the following compounds.
(a) 2 – Bromo pentane
(b) 2 – methyl propane
(c) butanol
(d) 1-hexine
Answer:
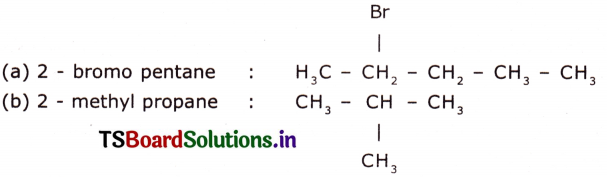
(c) butanol : CH3 – CH2 – CH – CHO
(d) 1 – hexine : CH3 – CH2 – CH2 – CH2 – C ≅ C – H
![]()
Question 6.
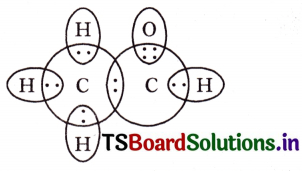
Draw the electron dot structures of Ethanoic acid and Ethyne (Acetylene)
Answer:
Ethanoic acid (Acetic acid):

Question 7.
Write the molecular formula of the first tour compounds of the homologous series of aldehydes.
Answer:
Homologous series of aldehydes are Formaldehyde, Acetaldehyde. Propionaldehycie arid Butanaidehyde.
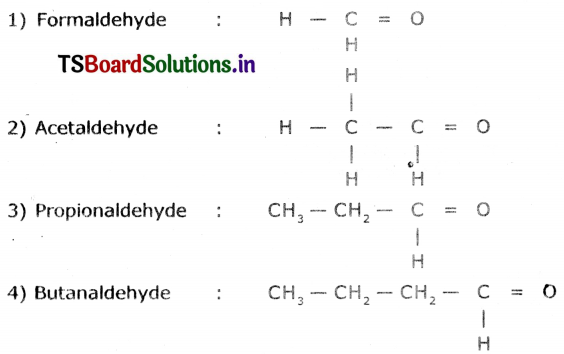
Question 8.
How many Isomers can be drawn for pentane with molecular formula C5H12? What are they? Draw their structures and mention their common names.
Answer:
Isomers of pentane are three. These are
- Pentane
- Iso pentane
- Neo pentane.
Structures:
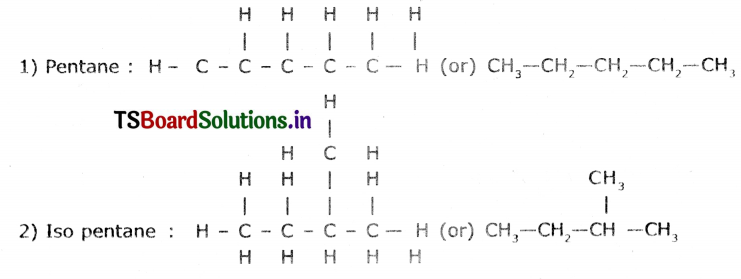
Question 9.
Explain sp3 hybridization In methane.
Answer:
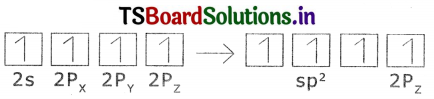
The excited carbon atom allows use S-orbital. (2s) and three p-orbitals (2px,
2py, 2pz) to intermix and reshuffle Into four identical orbitals known sp3 orbitals. Thus carbon atom undergo sp3 hybridization. The four electrons enter into the new four identical orbitals known as sp3 orbitals, one each as per Hund’s rule.
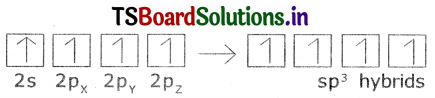
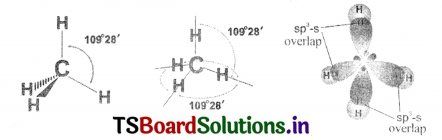
1. Since carbon has four unpaired electrons, t is capable of forming bonds with four other atoms.
2. When carbon reacts with hydrogen, four hydrogen atoms allow their ‘s’ orbitals containing one electron each to overlap with four sp3 orbitals of carbon-atom which are oriented at an angle of 109°. 28′.
3. Four orbitals of an atom in the outer shell orient along the four comers of a tetrahedron to have minimum repulsion between their electrons. The nucleus of the atom is at the centre of the tetrahedron.
4. This leads to form four sp3 – s – sigma bonds between carbon atom and four hydrogen atoms. All these bonds are of equal energy.
Question 10.
Explain sp2 Hybridisation with an example.
Answer:
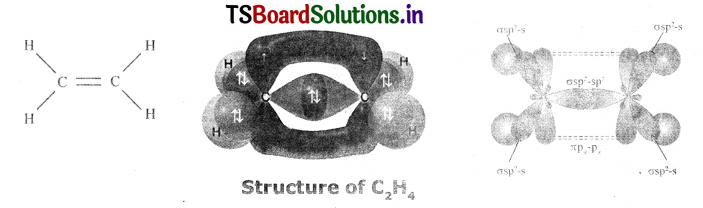
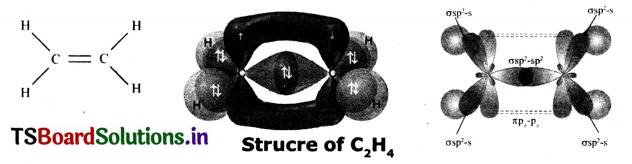
Consider ethene molecule
- In the formation of CH2 = CH2 each carbon atom in its excited State undergoes sp2 hybridization by Intermixing one s-orbital (2s) and two p-orbitals (say 2px and 2py) and reshuffling to form three sp2 orbitals.
- Now each carbon atom Is left with one ‘p’ orbital (say 2Pz) subsidized.
- The three sp2 orbitals having one electron each get separated around the nucleus of carbon atoms at an angle of 120°.
- When carbon Is ready to form bonds one sp2 orbital of one carbon atom overlaps the sp2 orbital of the other carbon atom to form sp2 – sp1 sigma (s) bond.
- The remaining two sp2 orbitals of each carbon atom get overlapped by ‘s’ orbitals of two hydrogen atoms containing unpaired electrons.
- The unhybridized pz orbitals on the two carbon atoms overlap laterally as shown in figure to form π bond.
- Hence, there exists a sigma (σ) bond and a pi (π) bond between two carbon atoms In ethene molecule. Hence, the structure of molecule ethane (C2H4)
Question 11.
Explain sp hybridisation.
Answer:
- Each carbon is only Joining to two other atoms rather than tour or three.
- Here the carbon atoms hybridise their outer orbitals before forming bonds, this time they only hybridise two of the orbitals.
- They use the ‘s’ orbital (2s) and one of the sp orbitals, by leave the other 2p orbitals unchanged.
- The new hybrid orbitals formed are called sp-hybrid orbitals because they are made by an s-orbital and a p-orbital reorganizing themselves.
Question 12.
Define substitution reaction and give an example
Answer:
A reaction in which one atom or a group of atoms in a given compound is replaced by other atom or group of atoms is called a substitution reaction. Alkanes undergo substitution reactions. Exampler Methane (CH4) reacts with chlorine in the presence of sunlight.
![]()
Question 13.
Give next Homologs of the following compounds and also give structure formula and name. (1) HCHO (2) CH3OH
Answer:
| Hornolog | Formula | Name |
| CH3CHO | C2H4O | Ethanal |
| CH3CH2CHO | C3H6O | Propanal |
| CH3CH2CH2CHO | C4H8O | Butanal |
| CH3CH2CH2CH2CHO | C5H10O | Pentanal |
Question 14.
Write the IUPAC names of the following compounds. (AS1)
i) CH3 – CH0 – CH2 – CH2 – CH2 – CH2 – CH2 – CH2CH2OH
ii) CH3 – CH2 – CH = CH2 – C ≅ CH
iii) CH3 – CH2– CH2– CH2 – CHO
iv) CH3 – CH2 – CH2 – CH2 – COOH
Answer:
1) nananol
2) 4 – ene 1 hexyne
3) pentanol
4) pentanoic acid
Question 15.
Complete the following reactions.
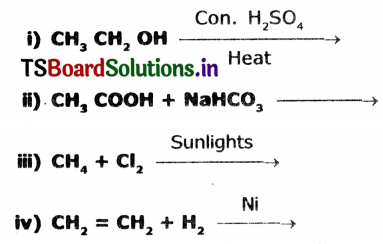
Answer:
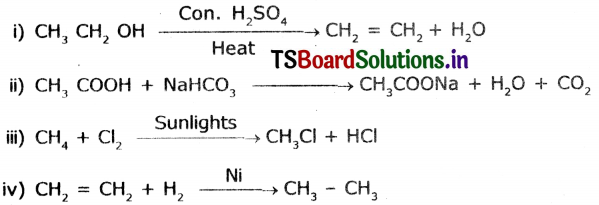
Question 16.
Draw the electronic dot structure of ethane molecule.
Answer:
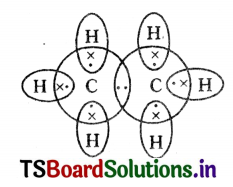
Question 17.
Draw the structure of the following compounds.
Answer:
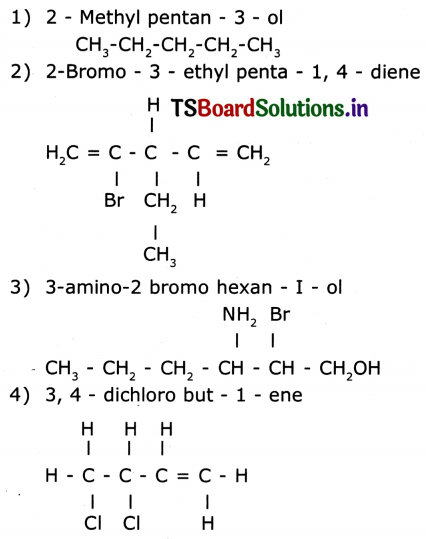
Question 18.
Draw various structures of (a) C5H12 (b) C6H14.
Answer:
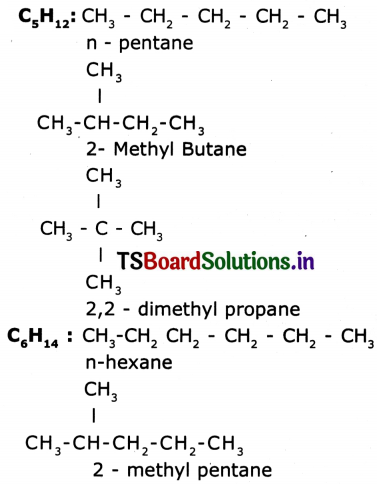
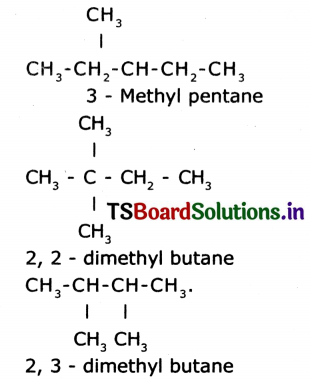
Question 19.
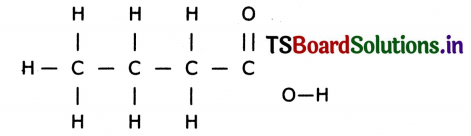
Draw the structuie of butanoic acid C3H7COOH.
Answer:
Formula of butanoic acid is C4H8O2.
Structure:
Question 20.
Draw the diagram showing sp2 hybridization In Ethene.
Answer:
Question 21.
Draw the diagram showing sp hybridisation in Ethyne.
Answer:
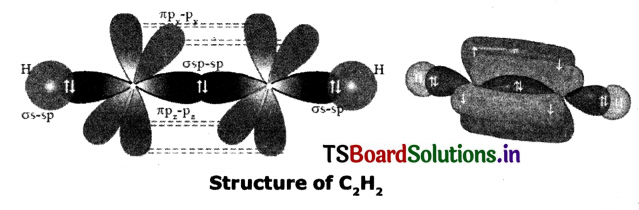
Question 22.
List out the materials required to conduct the experiment to understand the esterification reaction. Explain the procedure of the experiment. How can you identify that an ester is formed In this reaction?
Answer:
Materials required:
Test tube, beaker, water, burner wire gauze, tripod stand, 1ml. of ethanol, 1ml of glacial acetic acid, and few drops of conc. H2SO4
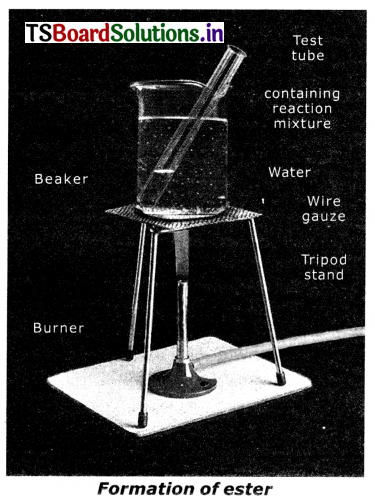
1) Take 1 ml of ethanol and imI of glacial acetic acid along with a few drops of concentrated sulphuric acid in a test tube.
2) Warm It In a water bath or a beaker containing water for at least 5 minutes as shown in fig.
3) Pour the warm contents into a beaker containing 20-50 ml of water and observe the odour of the resulting mixture.
Observation: The resulting mixture is a sweet odoured substance. This substance Is ester. This reaction is esterification, Ester is a sweet-smelling compound. We can identify an ester by its sweet smelling.
![]()
Do You Know?
1. Linus Pauling – The world’s one of the greatest scientist and a great humanist. He was acknowledged as the most influential chemist. He is the only person ever to receive two unshared Nobel Prizes – for Chemistry(1954) and for Peace (1962). (Page 255)

2. The buckminsterfullerene, or usually just fullerene for short, was discovered in 1985 by a team of scientists, Robert F. Curl, Harold W. Kroto, and Richard E. Smalley from Rice University and the University of Sussex, three of whom were awarded the 1996 Nobel Prize in Chemistry. They are named so for the resemblance of their structure to the geodesic structures devised by the scientist and architect Richard Buckminster “Bucky” Fuller. (Page 261)
3. Graphene: The new wonder material: As its name indicates, graphene is extracted from graphite, the material used in pencils. Like graphite, graphene is entirely composed of carbon atoms. For a thickness of 1mm, graphite contains some 3 million layers of graphene. The carbons are perfectly distributed in a hexagonal honeycomb formation only in 0.3 nanometers thickness. Graphene conducts electricity better than copper. It is 200 times stronger than steel but six times lighter. It is almost perfectly transparent to light. (Page 263)
4. Wohier Friedrich (1800 – 1882): German Chemist who was a student of Berzelius In attempting to prepare ammonium cyanate from silver cyanide and ammonium Chloride. he accidentally synthesized urea in 1828. This was the lust organic synthesis and shattered the vitalism theory.

Wohier pursued the matter further and discoiered that urea and ammonium cyanate had the same chemical fo.rnula, but very different chemical properties. This was an early discovery of Isomerism since urea has the formula CO(NH2)2 and ammonium cyanate has the formula NH4CNO. (Page 264)
5. We may compare amines to as we have done RON and R – 0 – R’ to H2O. If one Hydrogen Atom is replaced from NH3 by an alkyl group we get primary amines. If two hydrogen atoms of NH3 are replaced by two alkyl groups (same or different) we get secondary amines and if all the three hydrogen atoms are replaced by the same or different alkyl groups we get tertiary amines. (Page 269)
![]()
6. The police officer asks the suspect to blow air Into a plastic bag through a mouthpiece of the detecting instrument which contains crystals of potassium di-chromate (K2Cr2O7). As K2Cr2O7 is a good oxidizing agent, it oxidizes any ethanol in the driver’s breath to ethanoic acid, Orange Cr2O72- changes to bluish-green Cr3+ during the process of the oxidation of alcohol The length of the tube that turned into green is the measure of the quantity of alcohol that had been drunk. Nowadays police are using even an electronic Instrument containing small fuel cell that measures the electrical signal produced when ethanol is in the breath is oxidized. The police even use the IR Spectra to detect the bonds C – OH and C – H of CH3– CH2OH. (Page 283)