Telangana TSBIE TS Inter 1st Year Chemistry Study Material 2nd Lesson Classification of Elements and Periodicity in Properties Textbook Questions and Answers.
TS Inter 1st Year Chemistry Study Material 2nd Lesson Classification of Elements and Periodicity in Properties
Very Short Answer Type Questions
Question 1.
What is the difference in the approach between Mendeleev’s periodic law and the modern periodic law?
Answer:
Mendeleev’s periodic law is based on atomic weights while the modern periodic law is based on electronic configuration.
Mendeleev’s periodic law states that the physical and chemical properties of the elements are periodic functions of their atomic weight.
Modern periodic law states that the physical and chemical properties of the elements are periodic functions of their outer electronic configuration.
Question 2.
In terms of period and group, where would you locate the element with Z = 114?
Answer:
The electronic configuration of the element with Z = 114 is
1s²2s²2p 3s²3p63d10
4s²4p64d104f14 5s²5p65d105f14
6s²6p66d18 7s²7p²
Since in the atom of the element with Z = 114 electrons are filled in 7 orbits it should belong to 7th period. It has 4 electrons in the outermost orbit, so it should belong to IV A group.
Question 3.
Write the atomic number of the element, present in the third period and seventeenth group of the periodic table.
Answer:
The elements in 17th group are halogens. The halogen in the third period is chlorine. Its atomic number is 17.
Question 4.
Which element do you think would have been named by
a) Lawrence Berkeley Laboratory
b) Seaborg’s group
Answer:
a) Lawrencium (Z = 103)
b) Seaborgium (Z = 106)
![]()
Question 5.
Why do elements in the same group have similar physical and chemical properties?
Answer:
All the elements in a group have same outer electronic configuration which is responsible for the similar physical and chemical properties with some gradation.
Question 6.
What are representative elements? Give their valence shell configuration.
Answer:
s and p block of elements, excluding ‘O’ group are called ‘representative elements’. Their valence configuration is ns1-2np0-5.
Question 7.
Justify the position of f-block elements in the periodic table.
Answer:
- These are present in two series, 4f and 5f series. In these elements, the differ-entiating electron enters in the (n – 2)f sub-level.
- All these elements have similar outer configuration (n-2)f1-14 (n – 1)d0-1 ns². So all the 4f series and 5f series elements show similar properties.
For the above reasons, these elements are grouped together as lanthanides and actinides respectively and placed sepa-rately below the main body of the periodic table. These two series of elements belong to III B group only, in the 6th and 7th periods respectively.
Question 8.
An element ‘X’ has atomic number 34. Give its position in the periodic table.
Answer:
According to Bohr – Bury principle, the electrons are distributed in the various shells as : 2, 8, 18, 6. Total number of shells represents the period and the number of valence electrons represents the group. So it belongs to 4th period and VIth A group (Z = 34)
Question 9.
What factors impart characteristic properties to the transition elements?
Answer:
- Small size of atom
- High nuclear charge
- Variable valency and
- Availability of’d’ orbitals for bonding
Due to these reasons, transition elements exhibit characteristic properties.
Question 10.
Give the outer shells configuration of d- block and f-block elements.
Answer:
The outer shell configuration of d-block el-ements is (n – 1)d1-10 ns1 or 2.
The outer shell electronic configuration of f-block elements is
(n – 2)f1-14 (n – 1)d0 or 1 ns².
![]()
Question 11.
State and give one example for Dobereiner’s law of triads and Newland’s law of octaves.
Answer:
Dobereiner’s law of triads:
When a group of three elements of similar properties are arranged in increasing order of their atomic weight the atomic weight of the middle element is an arithmetic mean of the other two elements e.g.,
| Element | Atomic weight |
| Li | 7 |
| Na | 23 |
| K | 39 |
Newland’s law of octaves: When elements are arranged in the increasing order of their atomic weights, every eighth element has properties similar to the first element analogous to every eighth note that resembles the first in octaves of music.

Question 12.
Name the anomalous pairs of elements in the Mendeleev’s periodic table.
Answer:
In Mendeleev’s periodic table elements are arranged in the increasing order of their atomic weights. But few elements are arranged in reverse order. They are called anomalous pairs. They are Argon and Potassium, Tellurium and Iodine, Cobalt and Nickel.
Question 13.
How does atomic radius vary in a period and in a group? How do you explain the variation?
Answer:
As we go down in a group, atomic radius gradually increases.
Reason :
As we go down in a group, new shells are opened and electrons which enter in them are attracted weakly by the nucleus. As we go from left to right in a period, atomic radius gradually decreases.
Reason :
As we go along a period the electrons enter in the same outer shell but at the same time nuclear charge increases. Hence electrons are attracted strongly.
Question 14.
Among N-3, O-2, F–, Na+, Mg+2 and Al+3
a) What is common in them?
b) Arrange them in the increasing ionic radii.
Answer:
a) All the ions contain same number of electrons (10 electrons).
b) Al+3 < Mg2+ < Na+ < F– < O-2 < N-3
Question 15.
What is the significance of the term isolated gaseous atom while defining the ionization enthalpy.
Hint: Requirement for comparison.
Answer:
To compare the ionization enthalpy of different elements only isolated gaseous atoms must be considered.
![]()
Question 16.
Energy of an electron in the ground state of the hydrogen atom is – 2.18 × 10-18 J. Calculate the ionization enthalpy of atomic hydrogen in terms of J mol-1.
Answer:
Since the hydrogen atom is in ground state the electron is present in 1st orbit.
Ionization potential = E∞ – E1
= 0 – (- 2.18 × 10-18 J) = 2.18 × 10-18 J/atom
or 2.18 × 10-18 J × 6.023 × 1023
= 13.13 × 105 J / mole
Question 17.
Ionization enthalpy1 (IE1) of O is less than that of N-explain.
Answer:
In N, the 2p orbitals are half-filled (1s²2s² 2p¹x 2p¹y, 2p¹z) and more stable. Hence the IE1 of N is high. In O (1s²2s² 2p²x 2p²y, 2p²z), there are repulsions among the paired up 2p electrons, which lowers the I.E.
Question 18.
Which in each pair of elements has a more negative electron gain enthalpy?
a) O or F
b) F or Cl
Answer:
a) In a period from left to right electron gain enthalpy increases. So F has more electron gain enthalpy than O.
b) Due to small size of fluorine atom the electron repulsions are more. So some energy is required to overcome the repulsion. So electron gain enthalpy of chlorine is more than fluorine.
Question 19.
What are the major differences between metals and non-metals?
Answer:
1) Metals are usually solids at room temperature. Metals have high melting and boiling points. Metals are good conductors of heat and electricity. Metals are malleable and ductile.
2) Non-metals are usually solids or gases at room temperature. Non-metals have low melting and boiling points. Non-metals are poor conductors of heat and electricity. Most non metallic solids are brittle and are neither malleable nor ductile.
Question 20.
Use the periodic table to identify elements.
a) With 5 electrons in the outer subshell
b) Would tend to lose two electrons
c) Would tend to gain two electrons.
Answer:
a) The number of electrons in the outer orbit is equal to the group number of periodic table. Since the outer subshell contains five electrons it should belong to Vth A group.
b) Since the element tends to lose two electrons, its atom contains two electrons in the outer orbit. So the element belongs to second group.
c) The elements tend to gain two electrons to get octet to acquire stability. Since the element tends to gain electrons it should have 6 electrons in its outer orbit. So the element belongs to 6th A group.
![]()
Question 21.
Give the outer electronic configuration of s, p, d, and f-block elements.
Answer:
s – block elements ns¹ or ns²
p – block elements ns np¹ to ns² np6
d – block elements (n – 1)d1-10 ns1 or 2
f – block elements (n – 2)f1-14 (n – 1)d0 or 1 ns²
Question 22.
Write the increasing order of the metallic character among the elements B, Al, Mg, and K.
Answer:
B < Al < Mg < K
Question 23.
Write the correct increasing order of non-metallic character for B, C, N, F, and Si.
Answer:
More the electronegativity and ionization energies more is the non-metallic character. So the correct increasing order of non- metallic character is
Si < B < C < N < F
Question 24.
Write the correct increasing order of chemical reactivity in terms of oxidizing property for N, O, F and Cl.
Answer:
More the electronegativity and electron gain enthalpies more is the oxidation power.
N < O < F < Cl
Question 25.
What is electronegativity? How is this useful in understanding the nature of elements?
Answer:
The relative tendency of an atom in a covalent molecule to attract the shared pair of electrons towards itself is called electronegativity.
The elements having more electronegativity values are more non-metallic and the elements having less electronegativity values are more metallic.
Question 26.
What is screening effect? How is it related to IE?
Answer:
The electrons present in the inner shells act as screens between the nucleus and the valence shell electrons, in other words, these electrons partially neutralise the force of attraction of the nucleus, over the valence electrons. This is called, shielding or screening effect’.
When the screening effect increases (No. of inner electron-shells increase), the IP decreases.
The magnitude of screening effect ∝ \(\frac{1}{I.P}\).
![]()
Question 27.
How are electronegativity and metallic & non-metallic characters related?
Answer:
Electronegativity provides a means of predicting the nature of elements. Electronegativity is directly related to the non-metallic character of elements and inversely related to the metallic character of elements.
The electronegativity increases across a period from left to right. So metallic character decreases and non-metallic character increases.
In group electronegativity decreases from top to bottom. So the metallic character increases down and the non-metallic character increases.
Question 28.
What is the valency possible to arsenic with respect to oxygen and hydrogen?
Answer:
With respect to oxygen, the possible valency is 3 and 5 in As2O3 and As2O5 respectively. With respect to hydrogen, the valency of arsenic is 3 in AsH3.
Question 29.
What is an amphoteric oxide? Give the formula of an amphoteric oxide formed by an element of group – 13.
Answer:
The oxide which reacts both with acids and bases forming salts is called an amphoteric oxide. In 3rd group, Al2O3 and Ga2O3 are amphoteric oxides.
Question 30.
Name the most electronegative element. Is it also having the highest electron gain enthalpy? Why or Why not?
Answer:
The most electronegative element is fluorine (4.0). Fluorine do not have the highest EA value. Among halogens, Cl has the highest EA value. The EA values are in the order, Cl > F > Br > I > At.
Reason :
The size of F atom is small, compared to the size of Cl atom. The addition of one electron to F atom produces high electron density around it. Then the electron-electron repulsions increase in F atom. Because of this, F atom shows lesser tendency to attract another electron towards it and form F– ion. Hence F has low EA than that of Cl.
![]()
Question 31.
What is diagonal relation? Give one pair of elements, that have this relation.
Answer:
The first element of a group shows similarities in properties with the second element of the next group. This is called, diagonal relationship’.
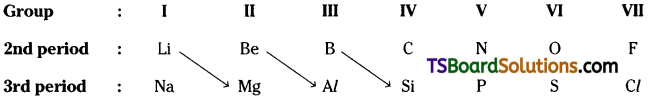
But this relation continues effectively upto the IV group only.
Question 32.
How does the nature of oxides vary in the third period?
Answer:
The basic nature decreases and the acidic nature increases from Na2O to Cl2O7, as shown below.

Question 33.
Radii of iron atom and its ions follow Fe > Fe2+ > Fe3+ – explain.
Answer:
When a neutral atom loses an electron, a positive ion (cation) forms. In this ion, there will be less number of electrons than protons. So the nuclear attraction on the valence electrons would increase. Then the size decreases.
As more and more electrons are removed, the nuclear attraction on the valence shell of electrons increases more and more. Consequently, the ionic size decreases more and more.
So, sizes : Fe > Fe2+ > Fe3+.
Question 34.
IE2 > IE1 for a given element – why?
Answer:
By the removal of an electron from a neutral atom, a uni +ve ion is formed. In this uni + ve ion the number of protons are more than the number of electrons. Hence the nuclear attractions will be more in uni + ve ion. As a result of which more amount of energy is required to remove an electron from the uni + ve ion. Hence, 2nd IP values are always greater than first IP values.
Question 35.
What is lanthanide contraction? Give one of its consequences.
Answer:
The steady decrease of atomic or ionic size from left to right in lanthanides, as the atomic number increases, is called Lanthanide contraction’.
In lanthanides, the differentiating electron enters the (n – 2) f subshell. Due to their peculiar shapes, f orbitals do not provided proper shielding for the valence electrons from the nuclear attraction. Consequently, the atomic or ionic size decreases gradually from left to right in lanthanides.
The decrease in contraction is more regular in Ln+3 ions than in Ln atom.
Consequences:
- Because of the lanthanide contraction, the hardness, m.p, b.p of the elements increase from Ce to Lu.
- There are more similarities between 4d and 5d series of elements than between 3d and 4d series. The main reason is, ‘lanthanide contraction’.
- The effect of lanthanide contraction is felt even in the post lanthanides. As a result, atomic sizes in the pairs of elements Zr/Hf; Nb/Ta; Mo/W are the same. So these pairs of elements possess similar properties. Zr and Hf resemble each other so much that their isolation is a quite difficult process.
![]()
Question 36.
What is the atomic number of the element, having maximum number of unpaired 2p electrons? To which group does it belong?
Answer:
A p orbital can accommodate maximum of 3 unpaired electrons. So the electronic configuration is 1s² 2s² 2p³. The atomic number of element is 7 and the element is nitrogen. It belongs to V th group.
Question 37.
Sodium is strongly metallic, while chlorine is strongly non-metallic – explain.
Answer:
Sodium is more electropositive due to its small ionisation energy. Hence it is strongly metallic.
Chlorine has highest electron affinity and also more electronegativity. So it is more non-metallic.
Question 38.
Why are zero group elements called noble gases or inert gases?
Answer:
Zero group elements have stable ns²np6 outer electronic configuration. Helium has completely filled 1s² configuration. So these elements are chemically inert and hence they are called inert gases. Recently it was found that these elements are also participating in chemical reactions but they are less reactive like noble metals such as gold and platinum. Hence they are also called noble gases.
Question 39.
Select in each pair, the one having lower ionization energy and explain the reason.
a) I and l–
b) Brand K
c)Li and Li+
d) Ba and Sr
e) O and S
f) Be and B
g) N and O
Answer:
a) I.P value of l– is less : Reason : The size of I– is greater than I
b) I.P value of K is less: Reason : K is electro +ve element whereas Br is electro -ve element
c) I.P value of Li is less : Reason : The size of Li is greater than Li+
d) I.P value of Ba is less : Reason: The size of Ba is greater than Sr
e) I.P value of S is less : Reason : The size of S atom is greater than 0 atom
f) I.P value of B is less : Reason : Be has fully filled atomic orbitals.
g) I.P value of 0 is less : Reason : N has half-filled atomic orbitals.
Question 40.
IE1 of O < IE1 of N but IE2 of O > IE2 of N – Explain.
Answer:
In N the 2p orbitals are half-filled (1s²2s² 2P¹xP¹yP¹z). The half-filled orbitals are more stable. In O (1s² 2s² 2p²x 2p¹x 2p¹y, 2p¹z) there are repulsions among the paired 2p electrons. So IP1 of O is less than N.
After removing one electron O+ has stable half-filled 2p³ electronic configuration but N+ has 2p² electronic configuration. So IE2 of O is greater than IE2 of N.
Question 41.
Na+ has higher value of ionization energy than Ne, though both have same electronic configuration – Explain.
Answer:
Na+ has more number of protons (11) than Ne (10). So the nucleus of Na+ holds the electrons strongly. Hence the ionisation energy of Na+ is greater than Ne.
![]()
Question 42.
Which in each pair of elements has a more electronegative gain enthalpy? Explain.
a) N or O
b) F or Cl
Answer:
a) Since N has stable halffilled p³ configuration it has less electron gain enthalpy than O.
b) Electron gain enthalpy of fluorine is less than chlorine because there is repulsion between electrons in small fluorine atoms.
Question 43.
Electron affinity of chlorine is more than . that of fluorine – explain.
Answer:
The size of fluorine atom is small. The addition of electron to small fluorine atom results in electron-electron repulsions. To overcome these repulsions some of the energy liberated due to addition of electron is consumed by fluorine. So the electron gain enthalpy decreases. These electron-electron repulsions are absent in bigger chlorine atom. So the electron gain enthalpy of fluorine is less than chlorine.
Question 44.
Which in each has higher electron affinity?
a) F or Cl–
b) O or O–
c) Na+ or F–
d)For F
Answer:
a) F
b) O
c) Na+
d) F
Question 45.
Arrange the following in order of increasing ionic radius:
a) Cl–, P-3, S-2, F–
b) Al+3, Mg+2, Na+, O-2, F–
c) Na+, Mg+2, K+
Answer:
a) F– < Cl– < S-2 < P-3
b) Al3+ < Mg2+ < Na+ < F– < O2-
c) Mg2+ < Na+ < K+
Question 46.
Mg+2 is smaller than O-2 in size, though both have same electronic configuration – explain.
Answer:
Mg2+ and O-2 are isoelectronic but Mg2+ has more number of protons (12) than O-2 (8). Due to more attractive power of nucleus of Mg2+ it is smaller than O-2.
Question 47.
Among the elements B, Al, C, and Si
a) Which has the highest first ionization enthalpy?
b) Which has the most negative electron gain enthalpy?
c) Which has the largest atomic radius?
d) Which has the most metallic character?
Answer:
a) C
b) C
c) Al
d) Al
![]()
Question 48.
Consider the elements N, P, O and S and arrange them in order of:
a) Increasing first ionization enthalpy
b) Increasing negative electron gain enthalpy
c) Increasing non-metallic character.
a) S < P < O < N
b) N < P < O < S
c) P < S < N < O
Question 49.
Arrange in given order:
a) Increasing EA : O, S and Se
b) Increasing IE1 : Na, K and Rb
c) Increasing radius: I–, I+ and I
d) Increasing electronegativity: F, Cl, Br, I
e) Increasing EA : F, Cl, Br, I
f) Increasing radius: Fe, Fe+2, Fe+3
Answer:
a) 0 < Se < S
b) Rb < K < Na
c) I– < I < I+
d) I < Br < Cl < F
e) I < Br < F < Cl
f) Fe+3 < Fe+2 < Fe
Question 50.
a) Name the element with highest ionization enthalpy.
b) Name the family with highest value of ionization enthalpy.
c) Which element possesses highest electron affinity?
d) Name unknown elements at the time of Mendeleef.
e) Name any two typical elements.
Answer:
a) Helium
b) Zero group
c) Chlorine
d) Eka Boron – Scandium
Eka Aluminium – Gallium
Eka Silicon – Germanium
Eka Manganese – Technisium
e) Sodium and magnesium.
Question 51.
a) Name any two bridge elements.
b) Name two pairs showing diagonal relationship.
c) Name two transition elements.
d) Name two rare earths.
e) Name two transuranic elements.
Answer:
a) Sodium and magnesium
b) Li, Mg; Be. Al
c) Chromium and Copper
d) Cerium and Lutesium
e) Neptunium and Plutonium
Short Answer Questions
Question 1.
On the basis of quantum numbers, justify that the 6th period of the periodic table should have 32 elements.
Answer:
The atoms of 6 th period elements contain 6 orbits containing the electrons.
In the long form periodic table, every period starts with the filling of a new orbit. So sixth period starts with the filling of principal quantum number 6. The sixth period should end with the filling of p-sub-shell of the same orbit. Thus the outer electronic configuration of last element is 6s²6p6. Before the 6p orbital starts filling the subshells to be filled with electrons are 6s, 4f and 5d.
The electrons that can be filled in 6s are 2, 4f are 14, 5d are 10 and 6p are 6, a total of 32 electrons. Thus the number of elements that can present in the 6th period are 32 .elements.
Question 2.
How did Mosley’s work on atomic numbers show that atomic number is a fundamental property better than atomic weight?
Answer:
Mosley (1913) obtained the X – ray spectrum of different elements and found that the frequency v of the lines in the spectrum is related with the atomic number (Z) of the element, as : √υ = a(Z – b)
a, b are constants for any selected series of lines in the X – ray spectrum (K, L, M, …… etc. series) of the element. The element is used as anticathode in the X – ray tube. Z = at. no. of the element (used as anticathode).
When Mosley plotted a graph between √υ × 108 (on Y – axis) and the atomic number Z (on X – axis) of the elements for which the X- rays were obtained, he got a straight line.
He also plotted a graph between √υ × 108 and atomic weight of the element. He did not get a straight line.
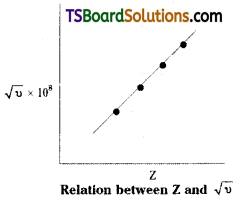
These observations indicated that atomic number is a fundamental property of an atom and not its atomic weight. Actually, the frequency (υ) of X – rays depends on the internal structure of the atom which is related to the number of electrons in the atom (atomic number).
Question 3.
State modern periodic law. How many groups and periods are present in the long form of the periodic table?
Answer:
Modern periodic law :
Modern periodic law was predicted by Mosley. It states that “The physical and chemical properties of the elements are periodic functions of their atomic numbers.”
Groups and periods :
In the long form of the periodic table given by Bohr, there are 18 groups (vertical columns) and 7 periods (horizontal rows).
![]()
Question 4.
Why are f-block elements placed below the main table?
Answer:
To accommodate the d-block elements the s-block and p-block elements are separated. There remains a gap between the s-block and p-block elements in the 2nd and 3rd periods.
If the f-block elements have to be accommodated in the periodic table again the size of the periodic table should be increased by separating the s-block and p-block. Then the table looks awkward. To avoid this the f-block elements are placed below the main table.
Question 5.
Mention the number of elements present in each of the periods in the long form periodic table.
Answer:
First period contains two elements only. Second and third periods contain eight elements.
Fourth and fifth periods contain eighteen elements only.
Sixth period consists of 32 elements. Seventh period is an incomplete period and contains 29 elements.
Question 6.
Give the outer orbit general electronic configuration of
a) Noble gases
b) Representative elements
c) Transition elements
d) Inner transition elements.
Answer:
General outer configurations :
a) Noble gases : ns²np6 (ns² for He)
b) Representative elements : ns1-2 np0-5
c) Transition elements : (n – 1)d1-10ns1-2
d) Inner transition elements : (n – 2)f1-14 (n – 1)d0-1ns².
Question 7.
Give any four characteristic properties of transition elements.
Answer:
Characteristic properties of d – block elements :
- They are hard and heavy metals.
- They possess high density, melting and boiling points.
- They are good electrical and thermal conductors.
- They exhibit variable oxidation states.
- Most of these are metals and their ions are paramagnetic.
- They and their ions exhibit colour.
- They and their oxides act as catalysts.
- They form alloys.
Question 8.
What are rare earths and transuranic elements?
Answer:
The 14 elements from cerium (Z = 58) to Lutesium are called rare earth elements because their abundance in the earth crust is very less. The properties of all these 14 elements are similar to lanthanum. So they Are called lanthanides or lanthanons or lanthanoids. These are called 4f series elements because in these elements the valence electron enters into 4f orbital.
The elements after uranium (Z = 92) in the periodic table are called transuranic elements. These elements do not occur in the nature. They are man made, synthetic and artificial. They are all radioactive and disintegrate into other elements. These elements are called 5f series elements, because in these elements the valence electron enters into 5f orbital.
Question 9.
What is isoelectronic series? Name a series that will be isoelectronic with each of the following atoms or ions.
a) F– b) Ar c) He d) Rb+
Answer:
Ions having the same number of electrons but different number of protons is called isoelectronic series. In isoelectronic series the size of the ion decreases with increases in atomic number.
a) N-3 O-2 F– Na+ Mg2+ Al3+
b) P-3 S-2 Cl– Ar K+ Ca2+ Sc3+
c) H– He Li+ Be2+
d) As3- Se2- Br– Rb+ Sr2+
Question 10.
Explain why cation is smaller and anion is larger in radii than their parent atoms.
Answer:
When an electron is removed from a neutral atom, cation is formed. The nuclear charge in both the cation and in its parent atom is the same. But the number of electrons in the cation are less than in its parent atom.
Hence, the nuclear attractions, in a cation will be more than in its parent atom. As a result of which the electron cloud of cat-ion shrinks. Hence the size of a cation is always smaller than its parent atom.
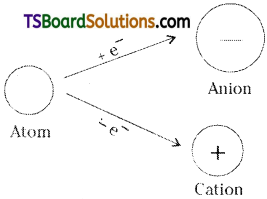
When an electron is added to a neutral atom, anion is formed. The nuclear charge in both the anion and in its parent atom is the same. But, the number of electrons in the anion are more than in its parent atom. Hence the nuclear attractions, in the anion will be less than in its parent atom. As a result of which the electron Cloud expands. Hence, the size of anion is always greater than its parent atom.
Question 11.
Arrange the second period elements in the increasing order of their first ionization enthalpies. Explain why Be has higher IE, than B.
Answer:
Li < B < Be < C < O < N < F
In Be the electron to be removed is from 2s orbital whereas in B the electron to be removed is from 2p. The penetration of a 2s electron to the nucleus is more than that of 2p electron, thus 2s electron is strongly attracted by the nucleus than 2p electron. The 2s electron is shielded by only one orbital Is in Be but 2p electron is shielded by two orbitals Is and 2s. So the IE1 of Be is more than IE1 of B.
Question 12.
IE1 of Na is less than that of Mg but IE2 of Na is higher than that of Mg – explain.
Answer:
Sodium atom has only one electron in its outer orbit and by losing that electron it gets stability by acquiring octet. Further in the nucleus of sodium there are less number of protons than in magnesium. So nuclear attraction on the electrons in sodium is less than in magnesium. So IE1 of Na is less than IE1 of Mg.
In Na+ the outer orbit has stable octet. To remove the electron from stable octet more energy is required. But in Mg2+ there is one more electron outside the stable octet i.e., in 3s orbital. To remove the 3s electron the energy required is less. So IE2 of Na is greater than IE2 of Mg.
![]()
Question 13.
What are the various factors due to which the IE of the main group elements tends to decrease down a group?
Answer:
1) Atomic size :
With increase in the atomic size, the distance from the nucleus to the outer electrons increases. So the attraction of the nucleus on outer electrons decreases. Hence IE decreases.
2) Nuclear charge :
With increase in nuclear charge i.e., effective nuclear charge, attraction of the nucleus on the outer electrons increases. So IE in-creases.
3) Screening effect or Shielding effect :
The inner orbits shield the nuclear attraction on the outer electrons. So with increase in the inner electrons shielding effect increases and thus IE decreases.
4) Extent of penetration of valence shell into inner electron :
The penetrating power of the orbitals towards the nucleus is in the order s > p > d > f. Nuclear attraction on the electrons in these orbitals also will be in the same order. So to remove an electron from different orbitals of the same orbit the energy required is in the order s > p > d
5) Number of charges on the ion :
With increase in the number of positive charges on an ion the nuclear attraction on the electrons increases. So IE increases.
6) Electronic configuration :
Atoms having octet in the outer orbit, or exactly half-filled and completely filled orbitals give stability to the atom. The energy required from the stable electronic configurations will be more.
Question 14.
The first ionization enthalpy values On k.J mol-1) of group 13 elements are :

How do you explain this deviation from the general trend?
Answer:
Generally I. P. values decrease from top to bottom in a group. Gallium (Ga) has more I.P. than Indium due to poor shielding effect of 3d electrons. Thallium has more l.P. than Indium due to poor shielding effect of 4f – electrons.
Question 15.
Would you expect the second electron gain enthalpy of oxygen as positive, more negative or less negative than the first? Justify.
Answer:
The second electron gain enthalpy of oxygen is always positive.
The amount of energy released when an electron is added to a neutral isolated gaseous atom is called electron gain enthalpy
X(g) + e– → X–(g) ; ΔH = -ve
As result of adding an electron to the neutral atom it converts into uninegative ion. It becomes difficult to add one more electron to this uninegative ion as there is repulsion between the negative charge on the ion and negative charge of electron. So it requires some energy to overcome the repulsion and to add an electron to the uninegative ion.
X–(g) + e– → X2-(g) ; ΔH = +ve
So the second electron gain enthalpy of oxygen is always positive.
Question 16.
What is the basic difference between the electron gain enthalpy and electropositivity?
Answer:
The amount of energy released when an electron is added to a neutral gaseous isolated atom is called electron gain enthalpy.
X(g) + e– → X–(g) ; ΔH = -ve
Electropositivity is the tendency to lose Electron. It is directly related to the metallic character. More the electropositive character of an element more the tendency to lose the electron and thus the element is more metallic in nature.
If electron gain enthalpy is more, the element has more tendency to gain electron. So that element will have more non-metallic character.
Elements having more electropositive character can act as strong reducing agents while the elements having more electron gain enthalpies will act as oxidising character.
Question 17.
Would you expect IE1 for two isotopes of the same element to be the same or different? Justify.
Answer:
Isotopes of the same element have same I.E values. Though the isotopes of an element have different atomic weights they have same atomic number. Since the nuclei of isotopes of same element contain same number of protons their nuclear charge is same. Also their atomic sizes are same. So the nuclear attraction on the electrons in different isotopes of same element is same. Hence the IE1 of the isotopes of the same element are same.
Question 18.
Increasing order of reactivity among group – 1 elements is Li < Na < K < Rb < Cs, whereas among group -17 elements it is F > Cl > Br > I – explain.
Answer:
Reactivity of group-I elements is proportional to metallic nature and metallic nature increases from Li to Cs. So, the order of reactivity is Li < Na < K < Rb < Cs.
Reactivity of group -17 elements is proportional to non-metallic nature. Non-metallic nature decreases from F to I. So, the order of reactivity is F > Cl > Br > I.
Question 19.
Assign the position of the element having outer electronic configuration.
a) ns²np4 for n = 3
b) (n – 1)d²ns² for n = 4
Answer:
a) n = 3 indicates the atoms of the element have electrons in three orbits. So the element belongs to third period.
Its outer electronic configuration ns²np4 indicates the presence of 4 electrons in its outer orbit. So the element belongs to VI(A) group of the periodic table as the number of electrons in the outer orbit is equal to group number. The element is silicon with outer electron configuration 3s² 3p².
b) n = 4 indicates the element belongs to 4th period as the number of orbits filled with electrons is equal to period number. Since there are two electrons (n – 1)d orbital it belongs to d-block elements. Its outer electron configuration is 3d²4s². The element is Titanium and it is in the IV(B) group of the periodic table.
![]()
Question 20.
Predict the formulae of the stable binary compounds that would be formed by the combination of the following pairs of elements.
a) Li and O
b) Mg and N
c) Al and I
d) Si and O
e) P and Cl
f) Element with atomic number 30 and Cl
Answer:
a) Valency of Li is 1 and that of O is 2. So the formula of compound is Li2G.
b) Valency of Mg is 2 and that of N is 3. So the formula of compound is Mg3N2.
c) Valency of Aluminium is 3 and that of I is 1. So the formula of the compound is AlI3.
d) The valency of silicon is 4 and that of oxygen is 2. So the formula of the compound is SiO2.
e) The phosphorous exhibits two types of valencies 3 and 5 but the compound with Cl in + 3 oxidation state is stable. So the formula of stable binary compound is PCl3.
f) The element with atomic number 30 is Zinc. Its valency is 2. So the formula of its binary compound with Cl is ZnCl2.
Question 21.
Write a note on the variation of metallic nature in a group and in a period.
Answer:
In any group of the periodic table as we move from top to bottom electropositive character increases. So metallic character increases.
In any period of the periodic table electronegativity increases. So metallic nature decreases.
Question 22.
How does the covalent radius increase in group 7?
Answer:
In every group, the differentiating electron enters in to a new orbit. So, the number of orbits in the atom of an element increases down the group. With the increase in the atomic number, the nuclear charge also increases, resulting in more attraction on the valence electrons. But the increase in the size of the atom exceeds the nuclear attraction. As a result the atomic radius (or) co-valent radius increases down the group.
Question 23.
Which element of 3rd period has the highest I.E1? Explain the variation of I.E1 in this period.
Answer:
In each period the last element i.e., the inert gas element has the highest I.E. In 3rd period, it is Argon.
Variation of I.E. in 3rd period :
The increasing order of IE of elements of 3rd period is Na < Al < Mg < Si < S < P < Cl < Ar. Mg and P have greater IE values than those of A1 and S respectively.
Reason:
In Mg, the 3s electrons are paired up (1s²2s²2p63s²). So the outermost electron i.e., 3s electron is paired up and moreover it is present in the s – orbital, which is more penetrating. So it requires more energy to remove this electron. So Mg has higher I.E., than expected.
In P, the 3p orbitals are half-filled (1s²2s²2p63s²3p¹x3p¹y3p¹z). So the atom is quite stable. So it. has higher I.E. than expected.
Question 24.
What is valency of an element? How does it vary with respect to hydrogen in the third period.
Answer:
Valency:
Valency is the combining capacity. It is the number of atoms of hydrogen or chlorine or any monovalent atom, with which one atom of the element combines.
Generally, all the elements in a group show the same valency. In case of s-block of elements, valency = group number.
In case of p – block of elements,
Valency = group number, or (8 – group no.)
Variation of valency w.r.t hydrogen, in a period :
In representative elements, the valency increases from 1 to 4 and then decreases to 1 from left to right.
Ex: Valency of 3rd period of elements :
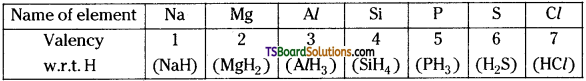
Question 25.
What is diagonal relationship? Give a pair of elements having diagonal relationship. Why do they show this relation?
Answer:
The first element of a group shows similarities in properties with the second element of the next group. This is called, ‘diagonal relationship’.
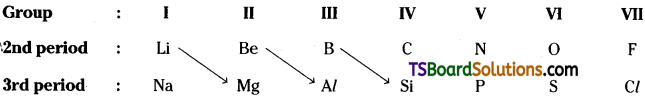
But this relation continues effectively upto the IV group only.
Question 26.
What is Lanthanide Contraction? What are its consequences?
Answer:
The steady decrease of atomic or ionic size from left to right in lanthanides, as the atomic number increases, is called ‘Lanthanide Contraction’.
In lanthanides, the differentiating electron enters the (n – 2) f subshell. Due to their peculiar shapes, f orbitals do not provide proper shielding for the valence electrons from the nuclear attraction. Consequently, the atomic or ionic size decreases gradually from left to right in lanthanides.
The decrease in contraction is more regular in Ln+3 ions than in Ln atom.
Consequences:
- Because of the lanthanide contraction, the hardness, m.p, b.p of the elements increase from Ce to Lu.
- There are more similarities between 4d and 5d series of elements, than between 3d and 4d series. The main reason is, lanthanide contraction’.
- The effect of lanthanide contraction is felt even in the post lanthanides. As a result, atomic sizes in the pairs of elements Zr/Hf; Nb / Ta; Mo/W are the same. So these pairs of elements possess similar properties. Zr and Hf resemble each other so much that their isolation is a quite difficult process.
![]()
Question 27.
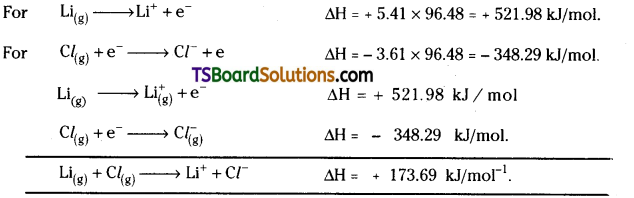
The first IP of lithium is 5.41 eV and electron affinity of Cl is – 3.61 eV. Calculate ∆H in kJ mol-1 for the reaction : Li(g) + Cl(g) → Li+(g) + Cl–(g)
Answer:
leV = 1.602 × 10-22 kJ/atom.
or leV / atom = 96.48 kJ/mol.
Question 28.
How many Cl atoms can you ionize in the process Cl → Cl+ + e by the energy liberated for the process Cl + e → Cl– for one Avogadro number of atoms. Given IP = 13.0 eV, and EA = 3.60 eV. Avogadro number = 6 × 1023.
Answer:
The energy liberated during the addition of electron to neutral isolated gaseous atom is electron affinity. For chlorine
Cl(g) + e– → Cl(g)– EA = 3.60 eV/atom
For one mole i.e., Avogadro number of chlorine atoms
3.60 × 6 × 1023 = 21.6 × 1023 eV.
The ionisation energy of chlorine atom
Cl → Cl+ + e+ is 13.0eV
The number of Cl atoms that ionise with 21.6 × 1023 eV is
\(\frac{21.6\times10^{23}}{13.0}\) = 1.662 × 1023
Question 29.
The electron affinity of chlorine is 3.7 eV. How much energy in kcal is released when 2g of chlorine atoms is completely converted to Cl– ions in the gaseous state? (1 eV = 23.06 kcal).
Answer:
1 eV / atom = 23.06 k cal / mol.
In the conversion of one mol. of Cl atoms into Cl– ions the energy liberated is 3.7 × 23.06 k.cal/mol.
The energy liberated by 35.5 g of Cl = 3.7 × 23.06 k.cal.
∴ The energy liberated by 2g of Cl = ?
\(\frac{2\times3.7\times23.06}{35.5}\) = 4.8069 k.cal.
Long Answer Questions
Question 1.
Discuss the classification of elements by Mendeleev.
Answer:
The periodic classification of elements bused on ‘atomic weights’ was done by Lothar Meyeer (Germany) and Mendeleev (Russia), independently.
Mendeleev’s periodic law:
“The physical and chemical properties of elements and their compounds are a periodic function of their atomic weights”.
Mendeleev arranged the then known 65 elements in a periodic table. He did not blindly follow the atomic weight but gave more importance to their chemical properties in arranging them in the table.
Explanation of the periodic law:
When the elements are arranged in the increasing order of their atomic weights, elements with similar properties appear again and again, at regular intervals, just like the days, weeks, months, seasons, etc. repeat at regular intervals of time. This is called periodicity of properties.
Mendeleev’s Table:
Mendeleev introduced a periodic table containing the then known 65 elements. In this table, while arranging the elements, he gave importance not only to their atomic weights, but also to their physical and chemical properties. This table was defective in some respects. Then he introduced another table, after rectifying the defects of that table. It is called, ‘Short form of periodic table’. He named the horizontal rows as ‘periods’ and the vertical columns, as ‘groups’. It has in all 9 groups, I to VIII and a ‘O’ group. The first 7 groups were divided into A and B subgroups. There are 7 periods in the table.
The VIII group contains three triads, namely, (Fe, Co, Ni); (Ru, Rh, Pd) and (Os, lr, Pt).
Merits of Mendeleev’s table :
- Actually, it formed the basis for the development of other modern periodic tables.
- Mendeleev left some vacant spaces in his periodic table, for the then unknown elements. But he predicted the properties of those elements. Later on, when these elements were discovered, they exactly fitted into those vacant places having properties, predicted by Mendeleev. Ex : Eka-boron (scandium), Eka-silicon (germanium), Eka-aluminium (gallium) etc.
- ‘O’ group elements were not known at the time of Mendeleev. Later when they were discovered, they found a proper place in that table under ‘O’ group of elements. Similarly, the radioactive elements.
- In case of these pairs of elements Tellurium – Iodine, Argon – Potassium and Cobalt – Nickel, there is a reversal of the trend. The first element has higher atomic weight than the second one. These are called, anomalous pairs.
However, based on their atomic numbers, and chemical properties, this arrangement proved quite justified.
Drawbacks of Mendeleev’s periodic table:
- Dissimilar elements were placed in the same group.
Ex : The coinage metals Cu, Ag and Au are placed along with the alkali metals K, Rb, Cs etc. in the I group. The only common property among them is that they are all univalent (valency = 1). - The 14 rare earths having different atomic weights are kept in the same place.
- Hydrogen could not be given a proper place, as it resembles both alkali met-als and halogens in its properties.
Question 2.
From a study of properties of neighbouring elements, the properties of an unknown element can be predicted – Justify with an example.
Answer:
Mendeleev left some gaps in the periodic table. He predicted the properties of these unknown elements after studying the properties of the neighbouring elements. When he proposed the periodic table, the elements scandium (Z = 21), gallium (Z = 31) and germanium (Z = 32) were unknown. Not only he predicted their properties but also named them eka-boron, eka-alumi- nium and eka-silicon respectively. The three elements were soon discovered within 20 years of his pronouncement of the ‘periodic law’ (during his life time). It is astonishing to see that when the properties of these elements were studied, these showed a remarkable agreement with those predicted by Mendeleev.
In addition to the above three elements, Mendeleev predicted the discovery of some more elements.
Examples:
| Properties of Eka-silicon predicted by Mendeleev (1871) | Properties of Germanium discovered by Winkler (1886) |
| 1. Atomic weight 72 | Atomic weight 72.6 |
| 2. Specific gravity 5.5 | Sp. gravity 5.46 |
| 3. Colour – dirty grey | Colour – greyish white |
| 4. Specific heat 0.073 | Specific heat 0.076 |
| 5. Oxide – ESO2 less basic than Sn O2, but more basic than Si O2 Sp. gr. 4.70, refractory | Oxide – Ge O2, slightly basic Sp. gr. 4.70, refractory |
| 6. Chloride – ESCl4 a liquid, B.P. below 100°C | Chloride – GeCl4, liquid B.P. 86.5°C |
![]()
Question 3.
Discuss the construction of long form periodic table.
Answer:
The important characteristic property of an element is found to be its ‘atomic number’ and not atomic weight. Accordingly, the periodic law was modified – “The physical and chemical properties of the elements are periodic functions of their atomic numbers”. Later on it was found that while deciding the properties of an element, its electronic configuration plays a very important role. So Bohr constructed the long form of the periodic table, based on the electronic configurations of the elements. The periodic law can be stated as “The physical and chemical properties of the elements are periodic functions of their electronic configurations”.
Salient features:
- This table is prepared based on a fun-damental property “atomic number”.
- This table can be easily studied, remem-bered and reproduced.
- Similarities, differences and trends in properties are more clearly reflected in this table.
- Vertical columns are known as groups and horizontal rows are called periods.
- There are seven periods in this table. The first period consists of two elements only. Second and third periods contain 8 elements each. Fourth and fifth periods contain 18 elements each. Sixth period consists of 32 elements. Seventh period is an incomplete period and consists of 19 elements.
- There are eighteen groups in this table. They are designated IA, IIA, IIIB, IVB, VB, VIB, VIIB, VIII, IB, IIB, 1IIA, IVA, VA, VIA, VILA, 0 (American Convention of naming).
- The elements in IA, IIA, IIIA, IVA, VA, VIA and VILA are known as representative elements or normal elements.
- The elements in IB, IIB, IIIB, IVB, VB, VIB, VIIB and VIII are called Transition elements.
- Zero group elements are placed at the extreme right of the table. These are called inert gases or noble gases. They possess stable ns np configuration.
- Short periods are broken and long periods are extended to accommodate transition elements.
- Lanthanides and actinides are placed separately at the bottom of the periodic table.
- Based on the entrance of differentiating electron, the table is divided into four blocks. They are s – block, p – block, d – block and f – block. In the elements of s – block, differentiating electron enters into s – orbital. Similarly in the elements of p – block, d – block and f – block, the differentiating electron enters into p, d and f – orbitals respectively.
- Based on complete and incomplete electron shells and chemical properties, the elements are classified into four types. They are 1) Type I (Inert gas elements) 2) Type II (Representative elements) 3) Type III (Transition elements) 4) Type IV (Inner transition elements).
- All the elements in a group possess similar properties, because they possess the same valence electron configuration.
Question 4.
Discuss the relation between the number of electrons filled into the sub energy levels of an orbit and the maximum number of elements present in a period.
Answer:
Construction of periods :
a) 1st period (short period) consists of two elements H1 and He2. The K shell (n = 1) can have a maximum of two electrons only. So there are only two elements in this period.
b) 2nd period (short period) contains 8 elements namely Li3 to Ne10.
Li atom has completed K – shell and a new shell, L starts with one electron in the remaining elements of the period i.e., from Be to Ne, the L shell is gradually filled up till Ne is reached. In Ne, the K as well as L shells are both completed.
Thus, the period ends. In these elements, the second energy level (L) gets gradually filled with a maximum of 8 electrons. So this period contains.8 elements.
c) 3rd period (short period) also contains 8 elements, i.e., Na11 to Ar18.
The M shell starts filling up with sodium. The shell builds up steadily, until argon is reached. After argon, the differentiating electron does not enter M shell (i.e., 3rd shell) but enters a new shell i.e., N shell (4th shell). Hence the 3rd period has only 8 elements.
d) 4th period (long period) has 18 elements which are K19 and Kr36.
The N shell starts filling in K (potassium), K has configuration 2, 8, 8, 1. In Ca, another electron enters the N shell. It has the configuration 2, 8, 8, 2.
Starting with the next element Sc (Z = 21), the penultimate M shell is expanded till it is complete with 18 electrons. In Cr and Cu, only one electron is present in the outermost shell i.e., Nth shell, while all the other elements contain 2 electrons. With Zn, the M shell is complete.
Then the extra electron enters the outermost (Nth) shell in the successive elements Ga to Kr. In this 4th period, 4s, 3d, 4p levels are successively filled with electrons. Hence this period has 18 elements.
e) 5th period follows closely the sequence in 4th period. It starts filling with 5s level. It ends when the 5p level is complete. The sublevels 5s, 4d and 5p get filled up. Then the total number of electrons filled into these levels is 18 and hence there are 18 elements in this period. The period starts with Rb and ends with Xe.
f) 6th period is long. In it, 6s, 4f, 5d and 6p energy levels get filled, which includes the 14 lanthanides. The total number of electrons filled in these levels is 32. Hence the total number of elements in the period is 32.
g) 7th period is incomplete, which includes the 14 actinides. There are nearly 20 elements in this period.
Question 5.
Write an essay on s, p, d and f block elements. [AP Mar. ’19; (AP, ’17, ’15; TS ’15]
Answer:
Depending upon the entering of differentiating electron into the atomic orbitals, the elements of long form of periodic table are divided into four blocks. They are s, p, d and f – blocks.
s – Block:
- The elements, in which the differentiating electron enters the s – sub-level are called s-block elements.
- In this block, there are two groups. They are IA and IIA.
- The valence electron configuration of elements of IA group is ns¹ and that of IIA is ns².
- The elements of LA are Alkali metals and IIA are Alkaline earth metals.
- These are metals and are highly reactive.
p – Block:
- The elements in which the differentiating electron enters the p – sub-level are called p – block elements.
- In this block, there are six groups. They are IIIA, IVA, VA, VIA. VIIA, and ‘O’.
- The valence – electron configuration of the elements of these groups varies from ns² np¹ to ns² np6.
- It includes metals, non-metals, metalloids, noble gases.
d – Block:
- The elements in which the differentiating electron enters into d – sub-level are called d – block elements.
- In this block, there are ten groups. They are IIIB, IVB, VB, VIB, VIIB, VIII, IB, IIB.
- The general outer configuration of d – block elements is (n – 1)d1-10 ns1-2.
- These elements are also known as Transition elements.
- These are all metals having high H.P and B.P.
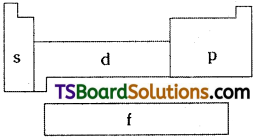
f – Block:
- The elements in which the differentiating electron enters into f – orbital are called f – block elements.
- In this block, there are 14 groups. They have no designation.
- Their general outer configuration is, (n – 2)1-14 (n – 1)d0-1ns².
- The f – block elements are arranged separately at the bottom in two rows.
- The elements of first row are called lanthanides and in these elements the valence electron enters into 4f orbital.
- The elements of second row are called actinides and in these elements the valence electron enters into 5f orbital.
- All the f – block elements are also known as Inner Transition elements.
- There are all metals.
Question 6.
Relate the electronic configuration of elements and their properties in the classification of elements.
Answer:
All the elements are divided into four types on the basis of their properties and electronic configurations. They are : (i) Noble gas elements (ii) Representative elements (iii) Transition elements and (iv) Inner transition elements.
i) Noble gas elements :
Elements in which the outermosts and p sub-shells are completely filled are called inert gas elements. “0” group elements belong to this type. Due to completely filled shells they show chemical inertness and possess more stability. The general outer configuration of these elements is ns²np6 (except helium.) The configuration of helium is 1s². They are called noble gases.
ii) Representative elements :
Elements in which the outermost s and p sub shells are incompletely filled are called representative elements. These elements are so named because they represent most of the chemical reactions known.
Elements of s – block and p – block (except ‘O’ group) belong to this type. The general outer electronic configuration of these elements is ns¹, ns², ns²np¹ to ns² np5. These elements enter into chemical reactions either by losing or gaining or sharing of electrons. Many of the non-metals, metalloids and some metals belong to this type.
iii) Transition elements:
Elements in which the outermost and penultimate shells are partially filled are called transition elements.
They belong to d-block. Their general configuration is (n – 1)d1-9 ns1-2. They are so named because there is a gradation from electro + ve nature to electro – ve nature. They possess the following characteristic properties.
- They exhibit variable oxidation states.
- They form coloured compounds.
- They are all paramagnetic.
- They and their oxides acts as catalysts.
- They form alloys and interstitial compounds.
- They form complex compounds.
iv) Inner transition elements:
Elements in which the outermost, penultimate and antipenultimate shells are partially filled are called Inner transition elements.
They belong to f – block and are placed separately at the bottom of the table. Their general outer configuration is (n – 2)f1-14 (n – 1)d0,1 ns1-2. They are so named as they represent a transition of physical and chemical properties among them. There are two series of inner transition elements corresponding to 4f and 5f series. 4f series are called lanthanides and elements of 5f series are called actinides. Lanthanides are also called rare earths. Majority of actinides are synthetic.
![]()
Question 7.
What is a periodic property? How the following properties vary in a group and in a period? Explain a) Atomic radius b) Electron gain enthalpy. [TS ’16, ’15; AP ’15; IPE ’14, ’11, ’10; Mar. ’18 (AP & TS)]
Answer:
The repetition of similar chemical properties of elements at regular intervals with increasing atomic number is called periodic property or periodicity.
a) Atomic radius :
In a period, as we go from left to right, the atomic radius gradually decreases.
Reason :
As we go from left to right in a period, the differentiating electron enters into the same orbit but at the same time, the nuclear charge increases. As a result of which the effective nuclear charge over the outermost electrons increases, leading to a decrease in the atomic radius.
As we go from top to bottom in a group, the atomic radius gradually increases.
Reason :
As we go from top to bottom in a group, the valence electrons enter into new shells, even though the nuclear charge increases. As a result of which, the effective nuclear charge over the outermost electrons decreases, leading to n increase in the atomic radius.
b) Electron gain enthalpy or Electron affinity :
In a period, as we go from left to right, Electron affinity gradually increases.
Reason:
As we go from left to right in a period, the atomic radius gradually decreases. As a result of which, the attraction between the added electron and the nucleus increases. Hence, electron affinity increases in a period.
In a group as we go from top to bottom, the electron affinity values gradually decrease.
Reason :
As we go down a group, the atomic radius gradually increases. As a result of which, the attraction between theadded electron and the nucleus decreases. Hence, electron affinity decreases in a group.
Question 8.
What is a periodic property? How the following properties vary in a group and in a period? Explain a) IP b) EN. [AP, TS ’16, ’15 ; IPE ’14, ’11, ’10; Mar. ’18 (AP & TS)]
Answer:
The repetition of similar physical and chemical properties of elements at regular intervals with increasing atomic number is called periodic property or periodicity.
a) Trends of I.P.:
As we go from left to right in a period, atomic radius gradually decreases. As a result of which the nuclear attractions on the valence electrons gradually increases. So, the energy required to remove the outer electrons gradually increases. In other words, as we go from left to right in a period, the I.P. values increase.
As we go from top to bottom in a group, atomic radius gradually increases. As a result of which the nuclear attraction on the valence electrons gradually decreases. So, the energy required to remove the outer electrons gradually decreases. In other words, in a group the I.P. values gradually decrease from top to bottom.
b) Electronegativity :
As we go from left to right in a period, the electronegativity gradually increases.
Reason:
In a period, as we go from left to right, atomic radius gradually decreases. As a result of which the tendency of attraction of nucleus on the bonded pair increases. Hence the values of electronegativity increases in a period.
As we go from top to bottom in a group, the electronegativity values gradually decrease.
Reason :
As we go from top to bottom in a group, the atomic radius gradually increases. As a result of which the tendency of attraction of nucleus on the bonded pair decreases. Hence, the values of electrone-gativity decreases in a group.
Question 9.
Write a note on
a) Atomic radius
b) Metallic radius
c) Covalent radius.
Answer:
Atomic radius refer to both metallic radius and covalent radius.
a) Atomic radius :
As the atomic radius increases, the distance between the nucleus and the outermost electrons increases. Hence, the effective nuclear charge on the outermost electrons decreases. As a result of which the energy required to remove the electrons decreases. From this it is evident that as atomic radius increases I.P decreases. Similarly as atomic radius decreases I.P increases.
b) Crystal radius (metallic radius) :
It is defined as one-half of the inter-nuclear distance between two adjacent atoms of a metal.
Ex : Crystal radius of sodium = \(\frac{3.72}{2}\) = 1.86 Å
Crystal radius of potassium = \(\frac{4.62}{2}\) = 2.31 Å
c) Covalent radius :
It is defined as one – half of the equilibrium distance between the nuclei of two atoms bonded by a covalent bond.
Ex: Covalent radius of hydrogen = 0.37 Å
Covalent radius of chlorine = 0.99 Å
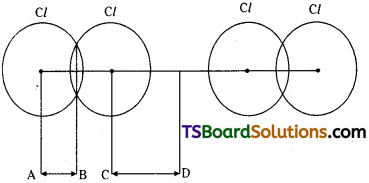
AB = covalent radius = 0.99 Å
CD = van der Waal’s radius = 1.8 Å
Covalent, van der Waal’s radii of chlorine
van der Waal’s radius:
It is defined as one-half of the inter-nuclear distance between atoms of two adjacent molecules of an element bonded by van der Waal’s forces facing each other, in solid state.
Ex : van der Waal’s radius of hydrogen = 1.2 Å
van der Waal’s radius of chlorine = 1.80 Å
Note:
van der Waal’s radius of an atom is 40% larger than its covalent radius.
![]()
Question 10.
Define IE1 and IE2. Why is IE2 > IE1 for a given atom? Discuss the factors that effect IE of an element. [TS Mar. ’19; AP, TS 16; Mar. ’13]
Answer:
The minimum amount of energy required to remove an electron present in the outermost orbit of a neutral, isolated gaseous atom is called ionisation potential. It is denoted by I1 and is measured in kilocalories or electron volts.
M(g) + I1 → M(g)+ + e–
The amount of energy required to remove another electron from a uni +ve ion is called second ionisation potential. It is denoted as I2.
M(g)+ + I2 → M(g)+ + e–
Ionisation energy is measured in ev/atom (or) k cal/mole (or) KJ/mole.
The uni +ve ion formed, by the removal of an electron from a neutral atom will have more nuclear attractions over the electron cloud because the number of protons will be more than the number of electrons. As a result of which more energy is required to remove an electron from this uni +ve ion than a neutral atom. Hence second I.P values are always greater than first I.P values.
Factors affecting Ionisation potential:
1. Atomic radius :
As the atomic radius increases, the distance between the nucleus and the outermost electrons increases. Hence, the effective nuclear charge on the outermost electrons decreases. As a result of which the energy required to remove the electrons decreases. From this it is evident that as atomic radius increases I.P decreases. Similarly as atomic radius decreases I.P increases.
2. Nuclear Charge :
As nuclear charge increases, the nuclear attractions over the valence electrons increases. So, more amount of energy is required to remove these electrons. From this it is evident that as nuclear charges increases I.P. increases. Similarly as nuclear charge decreases I.P. decreases.
3. Screening effect (or) Shielding effect:
In multielectron atoms, the electrons present in the inner shells screen the electron present in the outermost shells from the nuclear attractions. This effect is known as screening effect. Screening effect depends upon the number of screens (no. of inner orbits). As the screening effect increases, ionisation potential decreases. Similarly as the screening effect decreases, I.P. increases.
4. Extent of penetration of orbitals of valence electrons :
As the penetration power of orbitals increases, IP also increases. Order of penetration of orbitals is S > P > d > f. Hence, order of IP is also S > P > d > f.
5. Electron configuration :
Atoms of elements with half-filled or completely filled electron configuration are more stable. Such atoms have more IP values.
Question 11.
How do the following properties change in group – 1 and in the third period? Explain with example.
a) Atomic radius
b) IE
c) EA
d) Nature of oxides.
Answer:
a) Atomic radius :
As we move from top to bottom of group – 1 elements the atomic radius gradually increases. As we move from top to bottom in group -1 elements the number of orbits filling with electrons increases. So the atomic radius increases from top to bottom in group -1 elements.
In a period from left to right the atomic radius gradually decreases.
In the 3rd period, the differentiating electron enters into the same 3rd orbit. The electrons entering into the same orbit have less shielding power but the nuclear charge is increasing. Consequently the effective nuclear charge increases. So atomic radius decreases.
b) Ionisation Energy :
As we move from top to bottom of group -1 elements the atomic radius increases gradually. So the distance from the nucleus to the outer electrons increases. This results in the decrease in nuclear attraction on outer electrons. Hence ionisation energy decreases from Li to Cs as we move down the group.
While moving from left to right along 3rd period atomic radius decreases and effective nuclear charge increases gradually. So ionisation energy increases in the 3rd period from Na to Cl.
c) Electron Affinity :
As we move down the group -1 elements atomic radius increases gradually. Due to this reason the attraction between the added electron and the nucleus decreases. Hence electron affinity decreases from Li to Cs.
In the 3rd period while we are moving from left to right the atomic radius decreases and effective nuclear charge increases gradually. So the attraction between added electron and the nucleus increases gradually. Hence electron affinity increases from Na to Cl with certain exceptions.
d) Nature of oxides :
All the elements of 1A group are called alkali metals. Their oxides are basic in nature. They dissolve in water to give basic solutions.
Ex : Na2O, K2O etc.
Na2O + H2O → 2NaOH
The basic nature of these oxides increases from top to bottom in LA group.
In 3rd period the basic nature of oxides decreases and acidic nature increases from left to right.

Question 12.
Define electron gain enthalpy. How it varies in a group and in a period? Why is the electron gain enthalpy of O or F is less negative than that of the succeeding element in the group?
Answer:
The amount of energy released when an electron is added to a neutral isolated gaseous atom is called Electron affinity.
X(g) + e– → X–(g) + energy
As a result of adding up an electron to neutral atom, it gets converted into uni – ve ion. It is difficult to add up another electron to this uni – ve ion, because of the repulsions between the electrons already present and the electron to be added. Hence, in order to add up another electron, energy is to be given to overcome the repulsive forces. That is why second electron affinity values are always +ve.
X–(g) + e– → X––(g) + energy
In a period as we go from left to right, the electron affinity values gradually increases. In a group as we go from top to bottom, electron affinity values gradually decreases.
Electron affinity values of inert gases is ‘O’. The element with highest electron affinity value is chlorine.
Oxygen and fluorine atoms are smaller than their succeeding elements sulphur and chlorine. In oxygen and fluorine atoms the outermost orbit is second orbit when an electron is added to these small second orbit there will be electron-electron repulsions. To overcome these repulsions some energy is consumed from the energy liberated due to attraction on the added electron and nucleus. This results in the liberation of less energy or less negative electron gain enthalpies in 0 and F than that of the succeeding elements in the group.
Question 13.
a) What is electronegativity?
b) How does it vary in a group and in a period?
Answer:
a) The relative tendency of an atom in a covalent molecule to attract the shared pair of electrons towards itself is called its electronegativity’.
Pauling’s scale :
In this method, E.N. is calculated from the bond energies. The following expression is used.
XA – XB = 0.208 √∆
where XA = E.N. of atom A; XB = E.N. of atom B; ∆ = Bond polarity, where ∆ is in kCal/mole.
Bond polarity = Experimental bond energy – Theoretical bond energy.
∆ = EA-B – \(\frac{1}{2}\)(EA-A + EB-B)
where
EA-B = Bond energy of A – B molecule (experimental)
EA-A = Bond energy of A – A molecule
EB-B = Bond energy of B – B molecule
Metals are electropositive. So they possess low E.N. values. Non-metals are electronegative. So they possess high E.N. values.
b) In a group from top to bottom electronegativity decreases.
In a period from left to right electronegativity increases.
![]()
Question 14.
Explain the following
a) Valency b) Diagonal relationship c) Variation of nature of oxides in the Group -1.
Answer:
a) Valency :
Valency is the combining capacity. It is the number of atoms of hydrogen or chlorine or any monovalent atom, with which one atom of the element combines.
Generally all the elements in a group show the same valency. In case of s-block elements valency = group number.
In case of p – block of elements,
Valency = group number, or (8 – group no.)
Variation of valency w.r.t hydrogen, in a period :
In representative elements, the valency increases from 1 to 4 and then decreases to 1 from left to right.
Ex : Valency of 3rd period of elements :
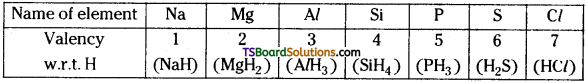
b) Diagonal relationship :
The first element of a group shows similarities in properties with the second element of the next group. This is called, diagonal relationship’.
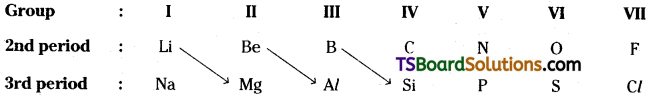
But this relation continues effectively upto the IV group only,
c) Variation of nature of oxides in the Group -1 :
All the elements of IA group are called alkali metals. These oxides are basic in nature. They dissolve in water giving alkaline solutions.
Na2O + H2O → 2NaOH
The basic nature of these oxides increases from top to bottom in group -1 elements.