Telangana SCERT TS 10th Class Physical Science Study Material Pdf 7th Lesson Classification of Elements- The Periodic Table Textbook Questions and Answers.
TS 10th Class Physical Science 7th Lesson Questions and Answers Classification of Elements- The Periodic Table
Improve Your Learning
I. Reflections on concepts
Question 1.
What are the limitations of Mendeleeff’s periodic table? How could the modern periodic table overcome the limitations of Mendeleeff’s table?
Answer:
Limitations of Mendeleeff’s periodic table: –
- Position of hydrogen: The position of hydrogen in table is not certain because it can be placed in group IA as well as in group VII A as it resembles both with alkali metals of IA group and halogens of VITA group.
- Anomalous pair of elements: Certain elements of higher atomic mass precede those with lower atomic mass. Eg: Tellurium proceeds Iodine, potassium placed after Argon. (Atomic mass 40) .
- DissimIlar elements placed together: Elements with dissimilar properties were placed in same groups as sub-group A and sub-group B.
Eg: U, Na, and K of the IA group have little resemblance with Cu, Ag, Au of IB group. - Some similar elements are separated: Some similar elements like copper and mercury ‘silicon and thallium’ etc., are placed ¡n different groups of the periodic table.
Question 2.
Define the modern periodic law. Discuss the construction of the long form of the periodic table.
Answer:
Modern Periodic Law: The physical and chemical properties of elements are the periodic function of the electronic configurations of their atoms.
- The modern periodic table has eighteen vertical columns known as groups and seven horizontal rows known as periods.
- The horizontal rows of elements In a periodic table are called periods. The elements in a period have consecutive atomic numbers. The vertical columns of elements In the table are called groups.
- Based on which subshell the differentiating electron enters In the atom of the given element the elements are classified as s, p. d, and f block elements.
s-Block elements :- The elements with valence shell configuration ns1 and ns2 are s- Block elements.
p-Block elements: The elements with electronic configuration from ns2np1 to ns2 np6 are p-block elements.
s and s block elements together are known as representative elements. d-Block elements or Transition elements:
The elements with valency electronic configuration ns2np6 nd1 to ns2np6 (n-1) d10 are called d-Block elements. These move from left to right in periodic table and we observe a transition of elements from metals to non-metals. Hence these are called transition elements.
f-Block elements or Inner transition elements: The elements In which f-orbitals are being filled in their atoms are called f-Block elements. These elements are called inner transition elements.
Inert Gases: Helium, Neon, Argon, Krypton, Xenon, Radon do not involve In any reaction and they are called Inert gases or noble gases. These are placed In zero group and every period ends with noble gas.
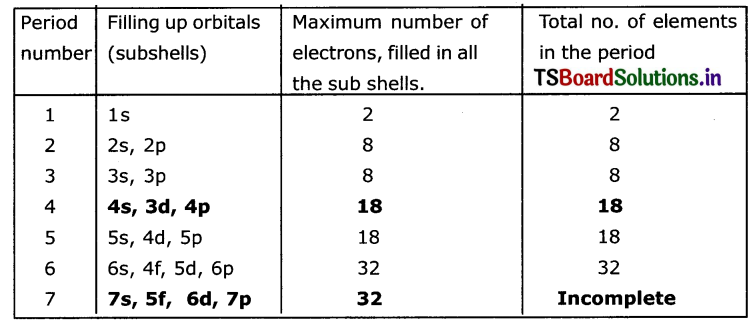
The long periodic table Is divided into 7 periods and 18 groups. First period contains 2 elements second period contains 8 elements. Third period contains 8 elements 4th and 5th periods contain 18 elements each and sixth period contains 32 elements. Seventh period is incomplete.
The 4f elements are called Lanthanolds. Elements from 58Ce to 71Lu. The 5f elements are called Actinoids from 90Th58 to 103 Lr 71 These are shown separately at the bottom of the periodic table.
![]()
Question 3.
Explain how the elements are classIfied Into s, p, d, and f-block elements In the periodic table and give the advantage of this kind of classification.
Answer:
Depending on the valency shell electronic configuration elements are classified into s, p, d, and f block elements.
s-Block elements: The elements with valence shell electronic configuration ns1 and ns2 are called s-block elements.
p-Block elements: The elements with valence shell electronic configuration ns2 np6 are called p-block elements. s and p block elements together known as Representative elements.
d-Biock elements : The elements with valence electronic configuration from ns2np6 (n -1)d1 to ns2np6(n -1)d10 are called d-block elements. These are also called as Transition elements.
f-Block elements: The elements In which f-orbitaIs are being filled in their atoms are called f – block elements. These are also known as Inner Transition Elements.
Advantage: This division of elements into blocks Is useful to divide the elements into groups. Every group has the elements with same valence electronic configuration. So they have similar chemical properties.
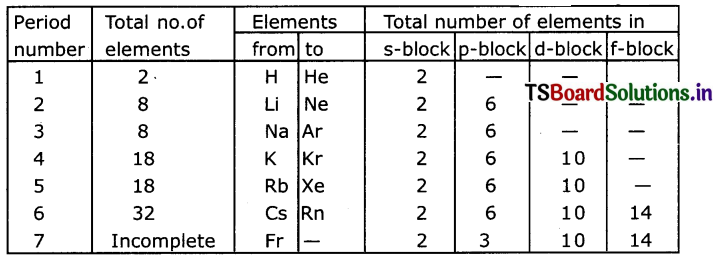
s-block elements – I A and II A groups
p-block elements – III A to VITI A or zero group.
d-block elements – I B to VIII B groups
f-block elements – Lanthanoids and Actinoids.
Question 4.
Write down the characteristics of the element having atomic number 17.
Electronic configuration …………………….
Period number …………………….
Group number …………………….
Element family …………………….
No. of valence electrons …………………….
Valency …………………….
Metal or Non-metal …………………….
Answer:
Electronic configuration: 1s22s22p63s23p5
Period number : 3
Group number: 17
Element family: Halogen (VII A) or 17th group
No. of valency electrons : 2+5 = 7 ( seven )
Valency: 1
Metal or Non-metal : Non-metal
Question 5.
Complete the following table using the periodic table.
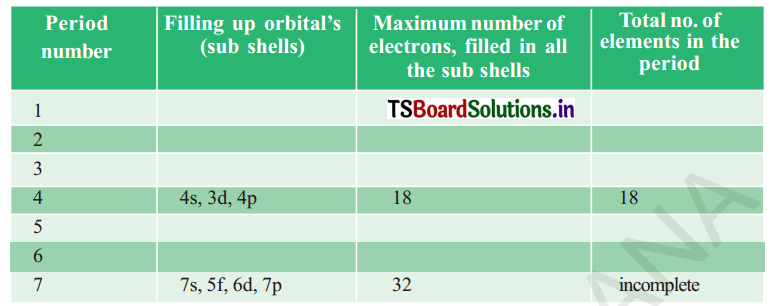
Answer:
Question 6.
Complete the following table using the periodic table.
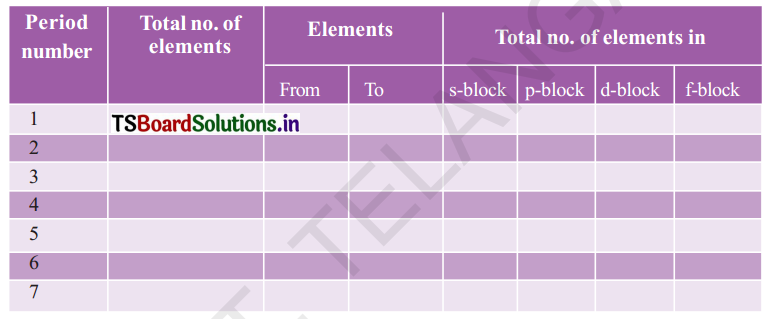
Answer:
Question 7.
Newlands proposed the law of octaves. Mendeleeff suggested eight groups for elements in his table. How do you explain these observations in terms of modern periodic classification?
Answer:
- Newlands proposed the law of octaves.
- According to Newlands law of octaves, every eight element starting from a given one be a repetition of the first with regard to its properties.
- Mendeleeff suggested eight groups for elements in his table.
- The elements in the same group have same properties that means every eight element starting from a given element have same property with that.
- In terms of modern concepts, after completion of one shell the properties of elements are repeated.
- After completion of ns2 np6 configuration the properties of elements are repeated.
- So Newlands law of octaves and Mendeleeff’s suggestion of eight groups for elements are also reliable according to modem concepts.
Question 8.
Why was the basis of classification of elements changed from the atomic mass to the atomic number?
Answer:
- The first attempt to classify elements was made by Dobereiner.
- Dobereiner’s attempt gave a clue that atomic masses could be correlated with properties of elements.
- Newlands’ law of octaves also followed the same basis for classification but this law Is not valid for the elements that had atomic masses higher than calcium.
- Mendeleeff’s classification also was based on the atomic masses of elements, but it lead to some limitations like Anomalous pair of elements and Dissimilar elements placed together.
- Mosley by analyzing the X-ray patterns of different elements was able to calculate the number of positive charges in the atoms of respective elements.
- With this analysis, Mosley realized that the atomic number is more fundamental characteristic of an element than its atomic weight.
- So, he arranged the elements in the periodic table according to the increasing order of their atomic number.
- This arrangement eliminated the problem of anomalous senes and dissimilar elements placed together In same group as done by Mendeleeff’s classification.
![]()
Question 9.
(a) What is a periodic property? How do the following properties change In a group and period? Explain.
(a) Atomic radius
(b) Ionization energy
(c) Electron affinity
(d) Electronegativity.
Answer:
Periodic property: Periodicity means that when elements are arranged according to their electronic configuration, the periodic properties of elements in the periodic table reoccur at regular intervals – such properties which reoccur are called as periodic properties.
Change of properties In a group and a period.
| Type of Periodic Property | Trends in | |
| Groups (from top to bottom) | Periods (from left to right) | |
| Atomic radius | Increasing | Decreasing |
| Ionization energy | Decreasing | Increasing |
| Electronegativity | Decreasing | Increasing |
| Electron Affinity | Decreasing | Increasing |
Explanation:
(a) Atomic radius: Atomic radius of an element Is not possible to measure in its isolated state, because it is not possible to determine the location of the electron that surrounds the nucleus. Hence the distance between nucleus and outermost orbit of an element is taken as atomic radius measured In Pm (Pico Meters).
Variation In group: In a group as we go down, the atomic number of the element Increases, as a new shell Is added. As a result, the distance between nucleus and the outer shell Increases. This Is the reason why the atomic radius increases as we go down in a group.
Variation In period: AtomIc radii of elements decrease across a period from left to right. As we go from left to right, electrons enter into the same main shell or even Inner shell. Therefore there should be no change In the distance between nucleus and outer shell, but nuclear charge Increases. Hence the nuclear attraction on the outer shell increases. As a result the size of the atom decreases.
(b) Ionization enegry: The energy required to remove an electron from the outermost orbit or shell of a neutral gaseous atom is called ionization energy.
Variation in group: As we go down in a group, atomic radius increases. Hence the ionization energy decreases from top to bottom.
Variation In period: As we go from left to right in a period, the atomic radius decreases. Hence the ionization energy increases from left to right In a period but does not follow gradient order as the electron is attracted more towards the nucleus.
(c) Electron affinity: The electron affinity of an element is defined as the energy liberated when an electron Is added to its neutral gaseous atom. Electron affinity of arm atom is also called electron gain enthalpy of that element.
Variation in group: Electron gain enthalpy values decrease as we go down In a group, due to Increase In atomic radius.
Variation In period: Electron gain enthalpy values Increase as we go from left to right in a period, due to decrease in atomic radius.
(d) Electronegativity: The electronegativity of an element Is defined as the relative tendency of its atom to attract electrons towards itself when it is bounded to the atom of another element.
Variation in groups and periods: Electronegativity values of elements decrease as we go down in a group and increase along a period from left to right.
Question 10.
(b) Explain the ionization energy order In the following sets of elements.
(a) Na, Al, Cl
(b) Li, Be, B
(c) C, N, O
(d) F, Ne, Na
(e) Be, Mg, Ca.
Answer:
(a) Na, Al, Cl: All these three elements belong to same period. The order of their atomic size Is Na > Al> Cl. As we move from left to right in a period Ionization energy increases
∴ The order of ionization energy of these elements is Cl> Al> Na.
(b) Li, Be, B: U, Be, B belong to same period. The electronic configuration of Li-1S22S1, Be-1S22S2, B-1S22S22p1. The penetration power of 2P is less when compared to 2s. So it is easy to remove electrons from ‘2p’.
∴ The order of ionization energy of these elements is: Be > Li> B.
(c) C, N, O : C, N, O belong to same period. The electronic configuration of C – 1s22s22p2, N-1s22s22p3, O-1s22s22p4. Nitrogen has half filled degenerate orbital configuration. So, it Is more stable compared to Cl and ‘O’so Its Ionisation energy Is high.
∴ Order of ionization energy of these elements is: N > C> O.
(d) F, Ne, Na: Electronic configuration of F -1S22s22p5; Ne – 1S22S22p6; Na – 1s22s22p63s1. Ne is an Inert gas, so It has highest Ionization energy.
‘Na’ has smaller size compared to F. So ¡t ha high ionization energy.
∴ The order of ionization energy is: Ne>F>Na.
(e) Be, Mg, CaP: These elements belong to the same group the atomic size of these elements Is In the order of Ca > Mg > Be. As atomic size increases ionization energy decreases. The order of Ionization energy Is Be > Mg > Ca.
Application Of Concepts
Question 1.
Given below Is the electronic configuration of elements A, B, C, and D.
(A) 1s2 2s2 1. WhIch are the elements coming within the same period?
(B) 1s22s22p63s2 2. Which are the ones coming within the same group?
(C) 1s22s22p63s2 3p3 3. Which are the noble gas elements?
(D) 1s22s22p6 4. To which group and period does the clement C ‘belong?
Answer:
(1) A and D belong to same penned B and C belong to the same period.
(2) A, B coming in the same group.
(3) D is the noble gas.
(4) C’ belongs to 15th group and third period.
Question 2.
s – block, and p – block elements (except 18th group elements) are sometimes called as ‘Representative elements’ based on their abundant availability In the nature. Is It Justified? Why?
Answer:
- s-block and p-block elements (except 18th group elements) are called ‘Representative elements’.
- All these elements have incomplete outermost shells.
- So they are chemically reactive to obtain stable electronic configuration of noble gases ns2np6.
- So, they are abundant In nature n the form of compounds.
Question 3.
The electronic configuration of the elements X, Y, and Z are given below.
(A) X = 2 (B) Y = 2, 6 (C) Z =2, 8, 2
(i) Which element belongs to second period?
Answer:
‘Y’ belongs to the second period. Because differentiating electron enters into second shell.
(ii) Which element belongs to second group?
Answer:
Element ‘Z’ belongs to second group. Because its valence is ‘2’.
(iii) Which element belongs to 18 group?
Answer:
Element X belongs to 18th” group because its 1st shell is completely filled with electrons.
![]()
Question 4.
(a) State the number of valence electrons, the group number and the period number of each element given in the following table.
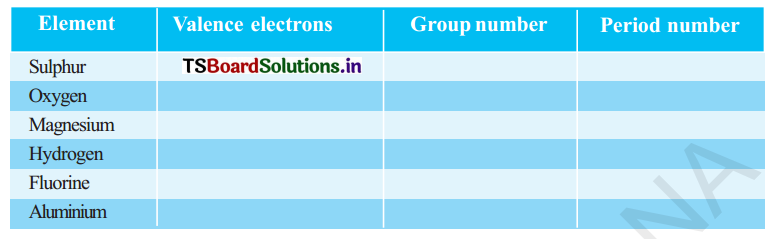
Answer:
| Element | Valence electrons | Group number | Period number |
| Sulphur | 6 | 16 (VIA) | 3 |
| Oxygen | 6 | 16(VLA) | 2 |
| Magnesium | 2 | 2 ( II A) | 3 |
| Hydrogen | 1 | No group | 1 |
| FluorIne | 7 | 17 ( VII A) | 2 |
| Aluminium | 3 | 13 (III A) | 3 |
(b) State whether the following elements belong to a Group (G), Period (P) or Neither Group nor Period Indicating the letters G or P or N
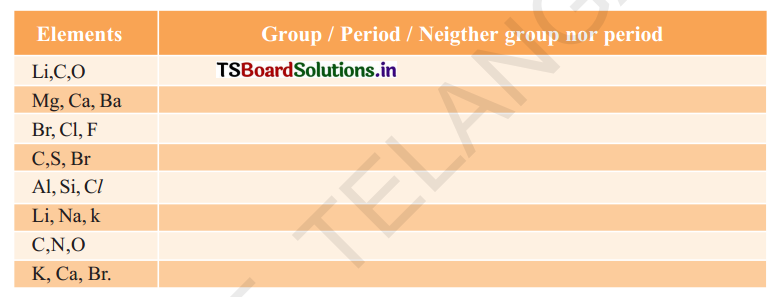
Answer:
| Elements | Group / Period / Neither group nor period |
| Li, C, O | P |
| Mg, Ca, Ba | G |
| Br, Cl, F | G |
| C, S, Br | N |
| Al, SI, Cl | P |
| Li, Na, K | G |
| C, N, O | P |
| K, Ca, Br | N |
Question 5.
Identify the element that has the larger atomic radius In each pair of the following and mark it with a symbol (✓).
(i) Mg, Ca
(ii) Li, Cs
(iii) N, P
(iv) B, AI
Answer:
- Mg, Ca: Calcium has large atomic radius. Since Mg and Ca are in same group Ca falling below Mg. Atomic radius increases from top to bottom In a group.
- Li, Cs: Cs has large atomic radius as Li and Cs are also in the same group and Cs lying below Li.
- N, P: Phosphorous has large atomic radius. As N and P are also In the same group and P lying below N.
- B, AI: Al has large atomic radius. B, Al are in same group III A and Al is below B.
Question 6.
Identify the element that has the lower Ionization energy in each pair of the following and mark it with a symbol (✓).
(i) Mg, Na
(ii) Li, O
(iii) Br, F
(iv) K, Br
Answer:
- Mg, Na: Na has low ionization energy. Since Mg, Na are in same period ionization energy increases from left to right. Na lies left to Mg. So Na has lower ionization energy.
- Li, O: Li has lower ionization energy. U lies left to ‘O’ in 2nd period.
- Br, F: Bromine has lower ionization energy; Br & F are in same group. IE decreases from top to bottom. Bromine is below F. So It has lower lE.
- K, Br: ‘K’ has lower ionization energy. Since it is left to Br in the same period.
Question 7.
How does metallic character change when we move
(i) Down in a group?
(ii) Across a period?
Answer:
(i) Down in a group?
Metallic character increases when we move down the group.
(ii) Across a period?
Answer:
Metallic character decreases when we move along a period.
Question 8.
On the bails of atomic numbers predict to which block the elements with atomic number 9, 37, 46 and 64 belong to?
Answer:
- Electronic configuration of element with atomic number 9 is 2, 7 – belongs to p-block.
- Electronic configuration of element with atomic number 37 is 2, 8, 8, 18, 1 – belongs to s- block.
- Electronic configuration of element with atomic number 46 is 2, 8, 8, 18, 10 – belongs to d – block.
- Electronic configuration of element with atomic number 64 Is 2, 8, 8, 18, 18, 10 -belongs tO f – block.
Question 9.
Using the periodic table, predict the formula of compound formed between an element X of group 13 and another element Y of group 16.
Answer:
Element X of group 13 Element Y of group 16
Valency of element X = 3 Valency of element Y
18-16=2

Compound: X2Y3
Question 10.
An element has atomic number 19. Where would you expect this element in the periodic table and why?
Answer:
The element with atomic number 19 is in 4th’ period and first group of periodic table.
Reason:
- Electronic configuration : Is2 2s2 2p6 3s2 3p6 4s1’.
- The differentiating electron enters Into 4t shell. Hence it belongs to 4th period.
- The differentiating electron is In ‘s’ orbital. So it belongs to ‘s’ block.
- The outermost orbital has only one electron. Hence it belongs to first group.
Question 11.
Elements In a group generally possess similar properties, but elements along a period have different properties. How do you explain this statement?
Answer:
- Physical and chemical properties of elements are related to their electronic configurations, particularly the outer shell configurations.
- The atoms of the elements in a group possess similar electronic configurations. Therefore, we expect all the elements in a group should have similar chemical properties and there should be a regular gradation in their physical properties from top to bottom.
- But in a period from left to right, elements get an increase in the atomic number by one unit between any two successive elements.
- Therefore, the electronic configuration of valence shell of any two elements in a given period is not same. Due to this reason elements along a period possess different chemical properties with regular gradation in their physical properties from left to right.
![]()
Question 12.
Name two elements that you would expect to have chemical properties similar to Mg. What is the basis for your choice?
Answer:
- Calcium (Ca) and Stranúum (Sr) are the two elements, which are similar to Mg in chemical properties.
- Because they belong to the same group lIA. Ail the elements which are in the same group have same electronic configuration and same chemical properties.
Question 13.
An element X belongs to 3rd perIod and group 2 of the periodic table. State (a) The no. of valence electrons in it (b) The valency (C) Whether It is metal or a non-metal.
Answer:
The element ‘X’ belongs to 3rd peñad and group 2 is Mg.
(a) The no.of valence electrons 2.
(b) The valency = 2.
(c) It is a metal.
Question 14.
How do you appreciate the role of electronic configuration of the atoms of elements In perIodic classification?
Answer:
- According to Modern Periodic Iaw the properties of elements are the functions of their atomic number or electronic configuration”.
- Elements having same number of valence electrons are placed in the same group based on their electronic configuration.
- So, we can easily locate the position of any element in the periodic table with Its electronic configuration.
- Hence electronic configuration plays a major role In the preparation of Modern period table. So its role is thoroughly appreciated.
Question 15.
Without knowing the electronic configurations of the atoms of elements Mendeleeff still could arrange the elements nearly close to the arrangements in the modern periodic table. How can you appreciate this?
Answer:
- Mendeleeff’s vision must be appreciable because he left some gaps predicting the existing of some elements, which are not available at that time.
- Atomic weights of some elements can be corrected by placing them In correct places.
- If we compare the long-form periodic table with Mendeleeff’s table, we find many elements with their places unchanged.
- One can see from these discussions, that the Mendeleeffs accurate prediction and their eventual success made him and his classification of elements famous, Even today his table of elements Is followed with minimum modifications.
Question 16.
Comment on the position of hydrogen in periodic table.
Answer:
- The configuration of Hydrogen (1s1) Is responsible for its dual nature.
- Either the electron can be lost behaving as electropositive elements (H+) like alkali metals or the electron can be gained as to complete, is subshell behaving as electronegative element (H–) like halogens.
- The position of Hydrogen is not fixed in the periodic table sometimes it is placed with alkali metals and sometimes with halogens.
- However, Hydrogen exhibits other properties which differ from both alkali metals and halogens.
Higher Order Thinking Questions
Question 1.
How does the positions of elements In the periodic table help you to predict Its chemical properties? Explain with an example.
Answer:
- The physical and chemical properties of atoms of the elements depend on their electronic configuration, particularly the outer shell configurations.
- Elements are placed in the periodic table according to the increasing order of their electronic configuration.
- The elements In a group possess similar electronic configurations. Therefore all the elements in a group should have similar chemical properties.
Eg: Consider K
- It is the element In 4th’ period 1st’ group.
- It is on left side of the periodic table. Hence It Is a metal.
- It is ready to lose an electron to get octet configuration. Hence its reactivity is more.
- It is Alkali metal.
- All alkali metals react with both acids and bases and release H2 gas.
Question 2.
In period 2, element X is to the right of element Y. Then find which of the elements have
(i) Low nuclear charge
(ii) Low atomic size
(iii) High Ionisation energy
(iv) High electronegativity
(v) More metallic character.
Answer:
X s to the right of Y. So they will be YX
(i) Low nuclear charge: Nuclear charge increases from left to right in a period. So Y has low nuclear charge.
(ii) Low atomic size: Atomic size decreases from left to right in a period. So X has low atomic size.
(iii) High ionization energy: Ionization energy increases across a period from left to right. So X has high ionization energy.
(iv) More metallic character: Metallic nature decreases from left to right. So Y has more metallic character.
![]()
Multiple choice questions
Question 1.
Number of elements present in period -2 of the long fom of periodic table ………………. [ ]
(a) 2
(b) 8
(c) 18
(d) 32
Answer:
(b) 8
Question 2.
Nitrogen (Z = 7) is the element of group VAofthe periodic table. Which of the following is the atomic number of the next element in the same group’? [ ]
(a) 9
(b) 14
(c) 15
(d) 17
Answer:
(c) 15
Question 3.
Electron configuration of an atom is 2,8,7. To which of the following elements would it be chemically similar? [ ]
(a) nitrogen(Z=7)
(b) fluorine(Z = 9)
(c) phosphorous(Z = 15)
(d) argon(Z=1 8)
Answer:
(b) fluorine(Z = 9)
![]()
Question 4.
Which of the following is the most active metal? [ ]
(a) lithium
(b) sodium
(c) potassium
(d) rubidium
Answer:
(d) rubidium
Suggested Experiments
Question 1.
Aluminium does not react with water at room temperature but reacts with both dil. HCl and NaOH solutions. Verify these statements experimentally. Write your observations with chemical equations. From these observations, can we conclude that Al is a metalloid?
Answer:
1. Aluminum reacts with dii. HCl and releases hydrogen gas with formation of Aluminium chloride.

2. Aluminium reacts with NaOH solution and releases hydrogen gas.
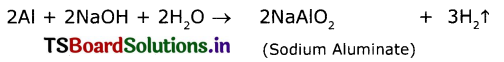
3. Aluminium does not react with water at room temperature. This concludes that the properties of Aluminium in between a metal and non-metal. So it behaves like a metalloid.
Suggested Projects
Question 1.
Collect the Information about reactivity of VIII A group elements (noble gases) from your school library and prepare a report on their special character when compared to other elements of periodic table.
Answer:
- The elements of Group VIII A (noble gases) are chemically inactive, because all of them have stable “Octet” configurations, except He.
- He has two electrons in the outermost orbital, i.e., is orbitalis is the maximum, the is orbital can accommodate. Hence “He” is also inactive.
- The losing or gaining an electron or sharing of electrons is difficult due to high I.E. and zero E.A value of inert gases.
- Under suitable conditions, the heavier elements of the group form compounds with F2 and O2 or XeF2 or XeO2F2.
- Ar forms coordination compounds with BF3 whose composition is Ar.n.BF3
- Xenon Hexa fluoro platinate (IV)(Xe[PtF6]) was prepared by N.Barltel. This was the first compounds of inert gases. After this compounds of Xenon with F2 and O2 were prepared.
- The compounds of inert gases have found varied applications in recent times.
Question 2.
Collect information regarding metallic character of elements of IA group and prepare report to support the idea of metallic character increases In a group as we move from top to bottom.
Answer:
Group IA elements are Li, Na, K, Rb, Cs, and Fr
1. Metallic character is the name given to the set of chemical properties associated with elements that are metals.
2. Chemical characteristics of metals include the following
- form cations in ionic compounds with non-metals.
- have ionic halides.
- have ionic hydrides containing the H’ ion.
- have basic oxides.
3. The elements with less electronegative character are metals.
4. All IA group elements have less electronegative characters because they are ready to lose one electron to get octet configuration.
5. If we observe this group, we can find that
Reaction with non-metals:
4M + O2 → 2M2O. In this cation is M+
Hydrides:
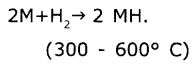
M stands for alkali metal.
Order of ionic nature of hydrides Is LiH < NaH < KH < RbH < CSH
Halides:
2M+X2 → 2MX
where M stands for alkali metal and X stands for halogen.
All the metal halides are ionic.
Oxides:
4M + O2 → 2M2O
But, 4 Li + O2 →‘ 2 Li2O2
All metals form peroxides.
6. From the above reactions we conclude that Group IA elements are metals and their metallic character increases as we go down in the group.
TS 10th Class Physical Science Classification of Elements- The Periodic Table Intext Questions
Page 124
Question 1.
Can you establish the same relationship with the set of elements given in the remaining rows?
Answer:
Yes, we can establish the same relationship with the set of elements given in the remaining rows.
Question 2.
Find average atomic weights of first and third elements ¡n each row and compare It with the atomic weight of the middle element.
Answer:
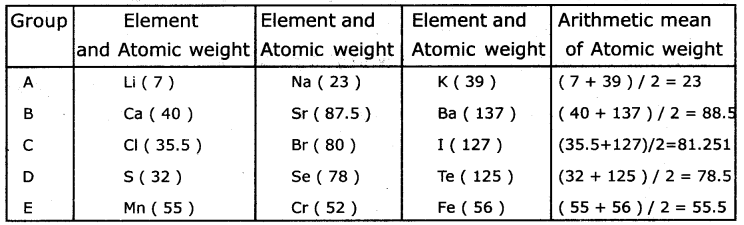
Question 3.
What do you observe?
Answer:
Dobereiner’s attempts gave a clue that atomic masses could be correlated with properties of elements.
Page 137
Question 4.
Find out the valencies of first 20 elements.
Answer:
| Element | Valency |
| 1.Hydrogen | 1 |
| 2. Helium | 0 |
| 3. Lithium | 1 |
| 4. Berflium | 2 |
| 5. Boron | 3 |
| 6. Carbon | 4 |
| 7. Nitrogen | 3 |
| 8. Oxygen | 2 |
| 9. Fluorine | 1 |
| 10. Neon | 0 |
| 11. Sodium | 1 |
| 12. Magnesium | 2 |
| 13. Aluminium | 3 |
| 14. Silicon | 4 |
| 15. Phosphorous | 3, 5 |
| 16. Sulphur | 2, 6 |
| 17. Chlorine | 1 |
| 18. Argon | 0 |
| 19. Potassium | 1 |
| 20. Calcium | 2 |
(b) How does the valency vary in a period on going from left to right?
Answer:
The valency increases from 1 to 4 and then decreases to zero as we move from left to right in a period with respect to Hydrogen. The valency increases from 1 to 7 with respect to oxygen, in a period from left to right.
(c) How does the valency vary on going down a group?
Answer:
The valency remains same in a group.
Page 139
Question 5.
Do the atom of an element and Its Ion have same size?
Answer:
No, the atom of an element and its ion do not have the same size. The size of cation is less than its neutral atom and size of anion Is greater than its neutral atom.
![]()
Question 6.
Which one between Na and Na would have more size? Why?
Answer:
- The atomic number of sodium is ti and It has 11 protons and ii electrons with outer electron as3s’ whereas Na4 ¡on has il protons but only 10 electrons.
- The 3s1 shell of Na has no electron In It.
- So the outer shell configuration is 2S2 2p6.
- As proton number is moie than electrons the nudeus of Na+ ion attracts outer shell electrons with strong nudear force.
- As a result the Na lori shrinks In size.
- Therefore, the size of Na ion is less than Na atom.
Question 7.
Which one between Cl and Cl– would have more size? Why?
Answer:
The electronic configuration of Cl is 1s22s22p63s23p5 whereas Cl– is 1s22s22p63s2 3p6. In Cl– , same no.of protons are attracting more number of electrons compared to Cl. Due to decrease in effective nuclear attraction atomic size of anion is more than its neutral atom.
Question 8.
Which one in each of the following pairs Is larger In size?
a) Na, AI
b) Na, Mg+2
c) S2, Cl–
d) Fe+2, Fe+3
e) C-4, F
Answer:
a) Na
b) Na
c) S-2
d) Fe+2
e) C-4
Think And Discuss
Question 1.
What relation about elements did Doberelner wanted to establish?
Answer:
The relative atomic mass of the middle element En each triad is nearer to the average of the relative atomic masses of other two elements. This is what Doberelner wanted to establish.
Question 2.
The densities of Calcium (Ca) and Barium (Ba) are 1.55 and 3.51gm/ cm3 respectively. Based on Doberolner’s law of triads can you give the approximate density of strontium (Sr)?
Answer:
The density of strontium = (1.55 + 3.51 )/2 = 5.06 / 2 = 2.53gm/cm3
Question 3.
Do you know why Newland’s proposed the law of octaves? Explain your answer In terms of the structure of atom.
Answer:
Newlands proposed the law of octaves. According to this law, every 8” element starting from a given elements resembles in its properties to that of starting element with the elements with similar chemical properties are to be present along horizontal.
In modem periodic table, every period starts with a new shell and ends when the shell is filled. We know about octet configuration. Newlands law of octaves Is similar to octet. configuration.
Question 4.
Do you think that Newlands law of octaves is correct? JustIfy.
Answer:
Newlands law of octaves is corretc. Mendeleeff also suggested only 8 groups in his table. Later it was discovered that eighth element has the same properties as the first one.
Question 5.
Why Mendeleeff had to leave certain blank spaces In his periodic table. What is your explanation for this?
Answer:
Based on the arrangement of the elements in the table, he predicted that some elements were missing and left blank spaces at the appropriate places In the table. Mendeleeff believed that some new elements would be discovered definitely. He predicted the properties of these new additional elements in advance purely depending on his table. His predicted properties were almost the same as the observed properties of those elements after their discovery.
Question 6.
What is your understanding about Ea2O3 and EsO2?
Answer:
Ea2O3 is the predicted formula of oxide,of the predicted element In eka – Aluminium group, This is similar to Ga2O3, the observed property of oxide of Gallum, which was discovered in 1875. EsO2 is the predicted formula of oxide of predicted element In eka-silicon group. This is similar to GeO2, the observed property of oxide of Germanium, which was discovered in.1886.
Question 7.
All alkali metals are solids but hydrogen is a gas with diatomic molecules. Do you justify the inclusion of hydrogen in first group with alkali metals?
Answer:
The similarity of hydrogen with alkali metals is ns’ configuration and ability to exhibit +1 oxidation state. Hydrogen, because of its small size and high electronegativity, it is a non-metal with high I.P. It shares one electron with another hydrogen and forms diatomic molecules. Due to weak van der Waals forces of attraction It is a gas whereas in alkali metals, metallic bond is present hence they are solids. Hence, hydrogen cant be included in first group along with alkali metals.
Question 8.
Why lanthanoids and actinoids placed separately at the bottom of the periodic table?
Answer:
Lanthanolds and actinoids are placed separately in the periodic table to maintain its structure and to preserve the principle of classification with similar properties in a single column.
Question 9.
If they are inserted within the table imagine how the table would be?
Answer:
If f-block elements are inserted within the table, the table would be too long.
Question 10.
Second Ionization energy of an element is higher than Its first ionization energy. Why?
Answer:
The required to remove first electron from the outermost orbit or shell of a neutral gaseous atom is called first ionization energy (IE1).
The energy required to remove an electron from uni-positive ion is called the 2nd ionization energy (IE2).
The second ionization energy (IE2) is greater than the first ionization energy (IE1). On removing an electron from an atom, the uni-positive ion formed will have more effective nudear charge than the neutral atom. This decreases the repulsion between the electrons and at the same time increases the nuclear attraction on the electron in the outer shells. As a result, more energy is required to remove an electron from the uni-positive ion. Hence the second ionization
energy is greater than the first ionization energy.
Question 11.
The calculated electron gain enthalpy values for alkaline earth metals and noble gases are positive. How can you explain this?
Answer:
Due to completely filled s-subshell in alkaline earth metals and completely filled valence shell (i.e. octet) in noble gases, they are highly stable. Hence energy Is required to add an electron I.e their electron gain enthalpy values are positive.
Question 12.
The second-period element, for example, F has less electron gaIn enthalpy than the third-period element of the same group for example ‘Cl’. Why?
Answer:
The atomic size of second period elements decreases and electron number increases as we move from left to right. Because of this elements of N, O, F possess vert small size and high electron density. Hence incoming electron feels repulsion due to existing electrons. Because of this, electron gain enthalpy
is less for second-period element compared to third period of the same group (In 5th’ , 6th and 7th groups).
TS 10th Class Physical Science Classification of Elements- The Periodic Table Activities
Activity 1
Question 1.
Observe the following table and till It.
Answer:
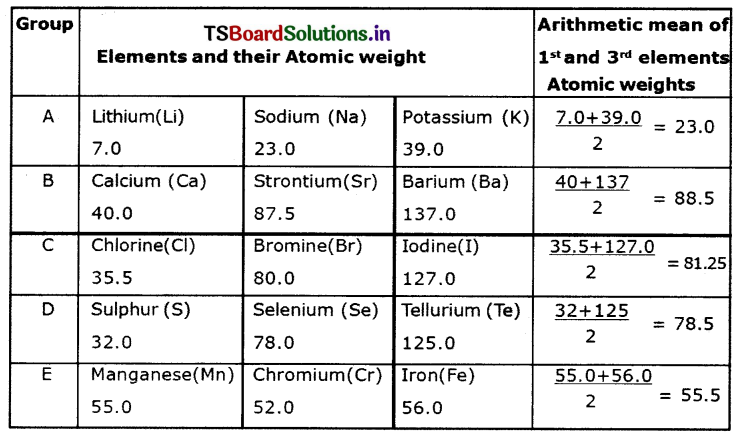
Observations:
a) Can you establish the same relationship with the set of elements given In the remaining rows?
Answer:
Except in the first row, we cannot establish the relationship as said by law of triads.
b) Find average atomic weights of first and third elements in each row and compare It with the atomic weights of the middle element.
Answer:
From table we observe that. except Group A, in the remaining Groups, the average of atomic weights of first and third elements is roughly equal to the middle elements.
c) What do you observe?
Answer:
Dobereiner’s attempt gave a clue that atomic mass could be correlated with properties of elements.
![]()
Activity 2
Question 2.
Write the different chemical families and write involved, valency of the family.
Answer:
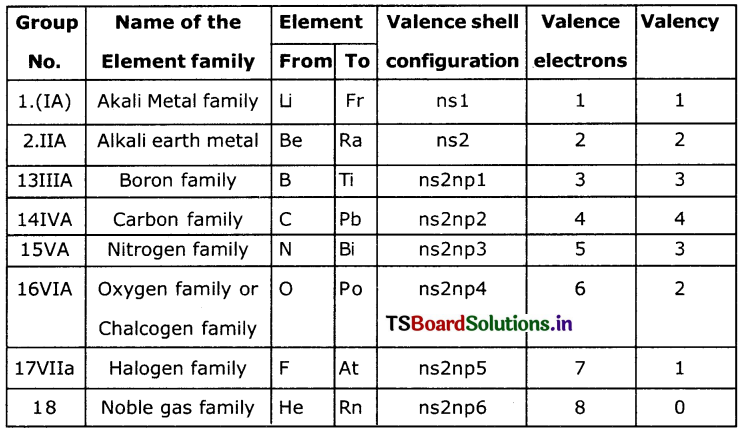
Question 3.
(a) Find out the valencies of first 20 elements.
(b) How does the valency vary in a period on going from left to right?
(c) How does the valency vary on going down a group?
Answer:
| Element | Valency |
| 1.Hydrogen | 1 |
| 2. Helium | 0 |
| 3. Lithium | 1 |
| 4. Berflium | 2 |
| 5. Boron | 3 |
| 6. Carbon | 4 |
| 7. Nitrogen | 3 |
| 8. Oxygen | 2 |
| 9. Fluorine | 1 |
| 10. Neon | 0 |
| 11. Sodium | 1 |
| 12. Magnesium | 2 |
| 13. Aluminium | 3 |
| 14. Silicon | 4 |
| 15. Phosphorous | 3, 5 |
| 16. Sulphur | 2, 6 |
| 17. Chlorine | 1 |
| 18. Argon | 0 |
| 19. Potassium | 1 |
| 20. Calcium | 2 |
(b) How does the valency vary in a period on going from left to right?
Answer:
The valency increases from 1 to 4 and then decreases to zero as we move from left to right in a period with respect to Hydrogen. The valency increases from 1 to 7 with respect to oxygen, in a period from left to right.
(c) How does the valency vary on going down a group?
Answer:
The valency remains same in a group.