Telangana SCERT TS 10th Class Physical Science Study Material Pdf 11th Lesson Principles of Metallurgy Textbook Questions and Answers.
TS 10th Class Physical Science 11th Lesson Questions and Answers Principles of Metallurgy
Improve Your Learning
I. Reflections on concepts
Question 1.
List three metals that are found In nature as Oxide ores.
Answer:
Zinc, Ferrous, Aluminium, and Magnesium are metals which are found in nature as oxide ores.
They are :
| Oxide ore | Metal | Formula |
| Zincite | Zinc | ZnO |
| Haematite | Ferrous | Fe2O3 |
| Bauxite | Aluminium | Al23O2H2O |
| Magnetite | Magnesium | MgCO3 |
Question 2.
List three metals that are found in nature n uncombined form.
Answer:
- Gold
- Platinum
- Silver
- Copper
Question 3.
Write a note on dressing of ore in metallurgy.
Answer:
- Dressing is the first step in extraction of metals.
- Ores that are mined from earth are usually contaminated with impurities such as soil and sand etc.
- Dressing means, simply getting rid of as much of the unwanted rocky material as possible before the ore is converted into the metal.
- Physical methods are used to enrich the ore.
- These methods adopted in dressing the ore depend upon difference between physical properties of ore and gangue.
- The following physical methods involved in dressing are 1) Hand picking 2) Washing 3) Froth floatation 4) Magnetic separation.
Question 4.
How do metals occur in nature? Give examples to any two types of minerals.
Answer:
The earth’s crust is the major source of metals. Sea water also contains some soluble salts such as sodium chloride and magnesium chloride etc. Some metals like Gold (Au), Silver (Ag) etc., are available in nature in a free state (native) as they are least reactive.
Other metals mostly are found in nature in the combined form due to their reactivity. The elements or compounds of the metals which occur in nature ¡n the earth’s crust are called ‘minerals’. Minerals in oxide form: Bauxite, Zincite, Magnetite, etc.
![]()
Question 5.
What is the difference between roasting and calcination? Give one example for each.
Answer:
| Roasting | Calcination |
| 1. Roasting is a pyrochemical process in which the ore is heated in the presence of air below its melting | 1. Calcination ¡s a pyrochemical process in which the ore is heated in the absence of air. |
| 2. The product is metal oxide obtained from sulphide ore. | 2. The product is metal oxide, obtained by decomposition of ore. |
| 3. Eg: 2ZnS + 3O2 2ZnO +2SO2 | 3. Eg : CaCO3CaO+CO2 |
Question 6.
Draw the diagram showing i) Froth floatation ii) Magnetic separation.
Answer:
i) Froth floats tian
ii) Magnetic separation
Question 7.
Draw a neat diagram of the Reverberatory furnace and label it neatly.
Answer:
Question 8.
What is an ore? On what basis a mineral is chosen as an ore?
Answer:
Ore: A mineral from which a metal can be extracted economically and conveniently is called ‘ore’.
To choose a mineral as an ore the following are considered
- The percentage of the metal in that mineral.
- Whether metal can be profitably extracted from it or not.
- The convenience of extraction of metal.
Question 9.
Write the names of any two ores of iron.
Answer:
- Haematite – Fe2O3
- Magnetite – Fe3O4
Question 10.
How do metals occur In nature? Give examples of any two types of minerals.
Answer:
The earth’s crust is the major source of metals. Sea water also contains some soluble salts such as sodium chloride and magnesium chloride etc. Some metals like Gold (Au), Silver (Ag) etc., are available in nature in free state (native) as they are least reactive. Other metals mostly are found in nature in the combined form due to their reactivity.
The elements or compounds of the metals which occur in nature in the earth’s crust are called ‘minerals’.
Minerals in oxide form: Bauxite, Ziricite, Magnetite, etc.
Minerals in sulphide form: Copper iron pyrites, Gatena, etc.
Question 11.
Write short notes on froth floatation process.
Answer:
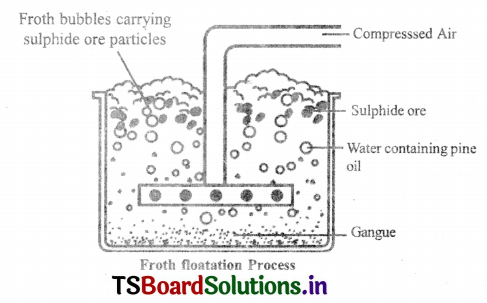
Froth Floatation process:
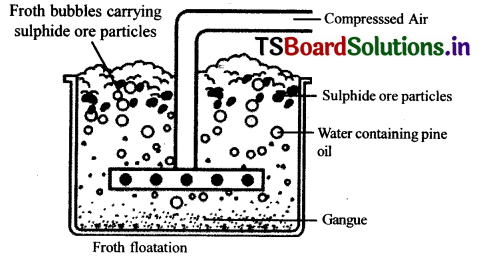
Froth Floatation process for the concentration of sulphide ores.
- This method is mainly useful for sulphide ores which have no wetting property whereas impurities get wetted.
- The ore with impurities is finely powdered and kept ¡n water taken in a floatation cell.
- Air under pressure is blown to produce froth in water.
- Froth so produced takes the ore particles to the surface whereas, impurities settle at the bottom.
- Froth is separated and washed to get ore particles.
Question 12.
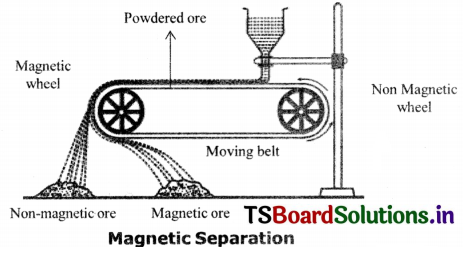
When do we use magnetic separation method for concentration of an ore? Explain with an example.
Answer:
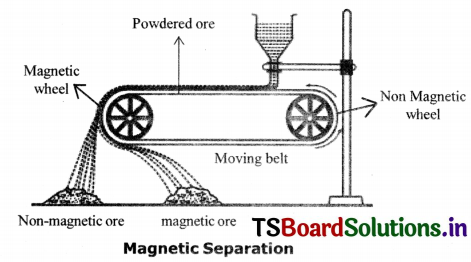
Magnetic Separation Method:
If the ore contains impurities such that one, of them, is magnetic and the other is non – magnetic they are separated by magnetic separation method.
Eg: Magnetic ores like iron pyrites, (FeS) and magnetite (Fe3O4) are concentrated by this method. The crushed ore is allowed to pass through electromagnetic belts. The mineral particles are retained and gangue particles are thrown away as a separate heap.
Question 13.
Write short notes on each of the following:
(i) Roasting.
(ii) Calcination.
(iii) Smelting.
Answer:
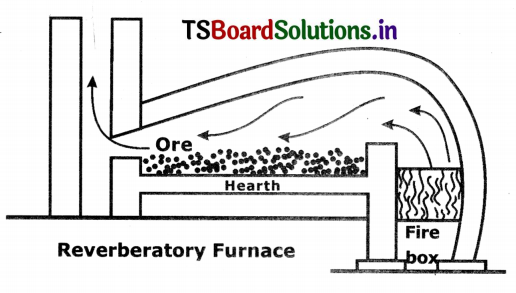
(i) Roasting:
- Roasting is a pyrochemical process in which the ore is heated in the presence of oxygen or air below its melting point.
- The products obtained in the process are also produced in solid state.
- Generally, reverberatory furnace is used for roasting.
Eg : 2ZnS + 3O2 → 2ZnO + 2SO2
(ii) Calcination:
- Calcination is a pyrochemical process in which the ore is heated in the absence of air.
- The ore gets generally decomposed n the process.
Eg: MgCO3 → MgO + CO2
(iii) Smelting:
- Smelting is a pyrochemical process ¡n which the ore is mixed with flux and fuel and strongly heated.
- The heat is so strong that the ore is reduced to even metal and the metal is obtained in molten state.
- During smelting, the impurities (gangue) in the ore react with flux to form slag which is removed.
- The smelting is carried out n a specially built furnace known as blast furnace.
Question 14.
What is gangue and slag?
Answer:
- Gangue: The impurity present in the ore is called gangue.
- Slag: A flux is a chemical substance added to convert gangue into fusible mass. This fusible mass is called slag.
Gangue + Flux Slag
Application of concepts
Question 1.
Magnesium is an active metal, if it occurs as a chloride in nature, which method of reduction is suitable for its extraction?
Answer:
Magnesium is an active metal. If it occurs as chloride in nature, the only method viable to extract magnesium ( any active metal) is electrolysis of fused Magnesium chloride. In electrolysis of fused Magnesium chloride, magnesium is deposited at cathode and chlorine gas is liberated at the anode.
MgCl2 → Mg+2 + 2Cl–
At cathode, Mg+2+ 2e– → Mg
At anode, 2Cl– → Cl2 + 2e–
Question 2.
Mention two methods which produce very pure metals from impure metals.
Answer:
Electrolysis and reduction are the two methods which produce pure metals.
![]()
Question 3.
Which method do you suggest for extracting of high reactivity metals? Why?
Answer:
High reactivity metals like K. Na, Ca, Mg, etc., can be extracted by electrolysis.
Reasons:
- Simple reduction methods like heating with C, CO, etc., to reduce the ores of these metals are not feasible.
- The temperature required for the reduction is too high and more expensive.,
- Hence electrolysis is the suggestable method to extract high reactive metals.
Question 4.
Explain Thermite process and mention its applications in our daily life.
Answer:
Thermite process
- When highly reactive metals such as Na, Ca, Al are used as reducing agents they displace metals of lower reactivity from their compounds.
- These displacement reactions are highly exothermic. The amount of heat evolved s so large that the metals produced will be in molten state.
- The reaction of iron oxide (Fe2O3), with aluminium is used to join railings of railway tracks or cracked machine parts. This reaction is known as the thermite reaction.
2Al + Fe2O3 → Al2O3 + 2Fe + Heat
Applications in daily life
1. To join railings of railway tracks.
2. Used to join cracked machine parts.
Question 5.
Where do we use handpicking and washing methods in our daily life? Give examples. How do you correlate these examples with enrichment of ore?
Answer:
We use hand picking in separating stones from rice and da I. We use washing methods to separate dust from rice, dal, vegetables, fruits etc.
Hand-picking: The colour and size of impurities is different from rice or dal. So we can easily separate them by hand-picking. In the same way if the ore particles and the impurities are n different sizes, colour etc., we can see this hand-picking method to separate ore from impurities.
Washing: Less-density particles like dust is separated from more density particles like rice, vegetables etc., by washing. In the same way ore particles are crushed and kept on a slopy surface. They are washed with a controlled flow of water. Less sensitive impurities are carried away by water flow, leaving the more sensitive ore particles behind.
Question 6.
What is activity series? How it helps in extraction of metals?
Answer:
Activity series: The arrangement of the metals in decreasing order of their reactivity is known as ‘activity series’.
Use of Activity series in extraction of metals
1. The method used for a particular metal for the reduction of its ore to the metal depends mainly on the position of the metal in the activity series.
Eg:
The metals at the top of the activity series (highly reactive) can be extracted by electrolysis.
The metals at the middle of the activity series can be extracted by
- reduction of metal oxide with carbon
- reduction of oxide ores with Co,
- self-reduction of sulphide ores
- reduction of ores with more reactive metals (thermite process).
3. The metals at the bottom of the activity series (less reactive) can be extracted by heating along, and displacement from their aqua solution.
Multiple choice questions
Question 1.
The impurity present in the ore is called as [ ]
(a) Ganguc
(b) fluid
(c) Slag
(d) Mineral
Answer:
(a) Ganguc
Question 2.
Which of the following is a carbonate ore? [ ]
(a) Magncsitc
(b) Bauxitc
(c) Gypsum
(d) tiaicna
Answer:
(a) Magncsitc
Question 3.
Which of the following is the corrcct formula of Gypsum [ ]
(a) CuSO4. 2H2O
(b) CaSO4. 1/2H2O
(c) CuSO4. 5H2O
(d) CaSO4. 2H2O
Answer:
(d) CaSO4. 2H2O
Question 4.
The oil used in the froth floatation process is [ ]
(a) kerosene oil
(b) pencil
(c) coconut oil
(d) olive coil.
Answer:
(b) pencil
Question 5.
Froth floatation is method used for the purification of …………………….. ore. [ ]
(a) sulphide
(b) oxide
(c) carbonate
(d) nitrate
Answer:
(a) sulphide
Question 6.
Galena is an ore of [ ]
(a) Zn
(b) Pb
(c) Hg
(d) Al
Answer:
(b) Pb
![]()
Question 7.
The metal that occurs in the native form is [ ]
(a) Pb
(b) Au
(c) Fc
(d) Hg
Answer:
(b) Au
Question 8.
The most abundant metal in th earth’s cruat is [ ]
(a) Silver
(b) Aluminium
(c) zinc
(d) iron
Answer:
(b) Aluminium
Question 9.
The reducing agent in thermite process is [ ]
(a) Al
(b) Mg
(c) Fe
(d) Si
Answer:
(a) Al
Question 10.
The purpose of smelting an ore is [ ]
(a) Oxidisc
(b) Reduce
(c) Neutndisc
(d) one of these
Answer:
(b) Reduce
Suggested Experiments
Question 1.
Suggest an experiment to prove that the presence of air and water are essential for corrosion. Explain the procedure.
Answer:
Corrosion: Corrosion is the deterioration of a metal, as a result of chemical reaction between it and the surrounding environment.
Experiment:
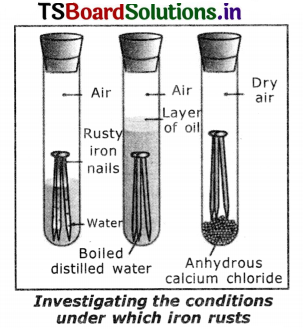
Aim: To prove that the presence of air and water are essential for corrosion. Materials required : 3 test tubes, 3 iron nails, oil, water, anhydrous calcium chloride, rubber corks.
Procedure:
1. Take three test tubes and place clean iron nails in each of them.
2. Label these tests tubes A, B and C. Pour some water in test tube A and cork it.
3. Pour boiled distilled water in test tube B, add about 1 ml of oil and cork it. The oil will float on water and prevent the air from dissolving in the water.
4. Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture.
5. Leave these test tubes for a few days and then observe.
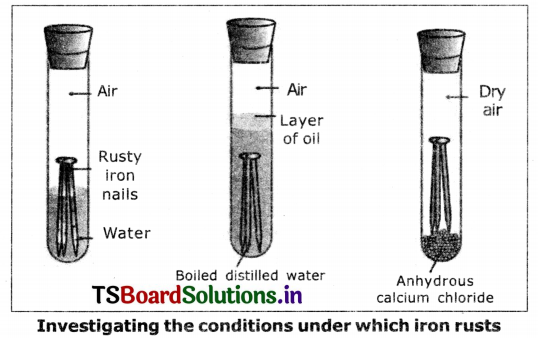
6. We will observe that ¡ron nails rust in test tube A, but they do not rust in test tubes B and C.
7. In the test tube A the nails are exposed to both air and water whereas in the test tube ‘B’ the nails are exposed to only water and in the test tube ‘C’ the nails are exposed to dry air.
8. This shows air and water are essential for corrosion.
Suggested Projects
Question 1.
Collect information about extraction of metals of low reactivity silver, platinum and gold and prepare a report.
Answer:
Extraction of Silver:
- Silver occurs both in combined state as well as In free state. The important ores of silver are Argentite (or) Silver glance (Ag2S) Pyrargyrite (or) Ruby silver 3 Ag2S Sb2S3 silver copper glance (CuAg)2S
- Silver is extracted from the ore-Argentite (Ag2S).
- The process of extraction of silver is called cyanide process, as sodium cyanide solution is used.
- The ore Is crushed, concentrated and then treated with sodium cyanide solution.
- This reaction forms sodium argent cyanide [Na[Ag(CN)2]]
Ag2S + 4 NaCN → 2Na[Ag(CN)2] + Na2S - This solution of sodium argent cyanide combines with zinc dust and forms tetra cyano zincate and precipitated silver. This precipitated silver is called spongy silver.
Zn + 2Na[Ag(CN)2] → Na2[Zn(CN)4)] + 2Ag - This spongy silver is fused with potassium nitrate to obtain pure silver. Then the silver obtained is purified by electrolytic process.
Extraction of platinum
- Platinum is rarely found on its own, but In combination with other base and precious metals.
- The extraction process of platinum is a complex process which includes milling the ore and smelting at high temperatures. This removes base metals notably sulphur and concentrates PGM (Platinum Group Metals) – Gold, Platinum and Palladium.
- The PGM matter is further processed by electrolysis to remove Nickel, Cobalt and Copper.
- The high-grade concentrate is treated by solvent extraction, distilling, and ion- exchange treatments to separate the PGMs Into its separated metals.
Extraction of Gold:
- Gold is usually found alone or alloyed with mercury or silver.
- In all methods of gold ore refining, the ore is usually washed and filtered at the mine, then sent to the mill. At the mill, the ore is ground into smaller particles with water, then ground again in a ball mill to further pulverize the ore.
- Several processes can be used to separate the Gold from its ore. They are:
a) Cyanide process:
- The ground ore Is put In a tank containing a weak cyanide solution and zinc is added.
- The zinc causes a chemical reaction which separates the gold from the ore.
- The gold is then removed from the solution with a filter press.
b) Carbon-in-pulp method:
- In this method, the ground ore is mixed with water before cyanide Is added. Then carbon is added to bond with the gold.
- The carbon-gold particles are put into a caustic carbon solution, separating out the gold.
c) Heap leaching:
- The ore is placed on open-air pads and cyanide is sprayed over It, taking several weeks to leach down to an imperious base.
- The solution then pours off and pad into a pond and is pumped from there to a recovery plant, where the gold is recovered.
- Heap-leaching helps recover gold from ore that would otherwise be to expensive to process.
TS 10th Class Physical Science Principles of Metallurgy Intext Questions
Page 237
Question 1.
Can you mention some articles that are made up of metals?
Answer:
Utensils in kitchen, window gnUs, pots, chairs, iron gates, bodies of motor cars, etc.
Question 2.
Do metals exist in nature in the same form as that we use ¡n our daily life?
Answer:
No, metals do not exist in nature ¡n the form same as that we use in our daily life.
Question 3.
Have you ever heard the words like ore, mineral and metallurgy?
Answer:
Yes.
![]()
Question 4.
Do you know how these metals are obtained?
Answer:
The metals are extracted from their ores mainly in three stages.
- The concentration of ore.
- Extraction of crude metal.
- Refining of the metal.
Question 5.
How the metals are present In nature?
Answer:
The metals are present in nature In combined form as their compounds.
Page 239
Question 6.
What metals can we get from the ore mentioned in Table-1?
Answer:
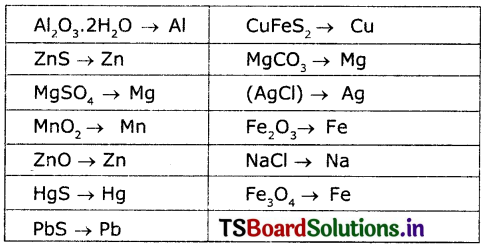
Question 7.
Can you arrange these metals in the order of their reactivity.
Answer:
K>Na>Ca>Mg>Al >Zn>Fe>Pb>Cu>Ag>Au
Question 8.
What do you notice In table-2?
Answer:
I noticed that the ores of many metals are oxides and sulphides.
Question 9.
Can you think how do we get these metals from their ores?
Answer:
These metals are obtained from their ores by suitable metallurgical processes.
Question 10.
Does the reactivity of a metal and form of its ore (oxides, sulphides, chlorides, carbonates, sulphates) has any relation with process of extraction?
Answer:
Yes, highly reactive metals are obtained by electrolysis from their molten salts, moderately reactive metals are obtained by reducing with suitable reagents whereas least reactive metals are available in native form.
Question 11.
How are metals extracted from mineral ores?
Answer:
Metals are extracted from mineral ores In three steps.
- Concentration of ore or Dressing.
- Extraction of crude metal.
- Refining of the metal.
Question 12.
What methods are to be used?
Answer:
Hand-picking, Washing, Froth floatation and Magnetic Separation methods are to be used.
Page 247
Question 13.
Do you know why corrosion occurs?
Answer:
Metals are stable in the form In which they are available in nature. So by means of corrosion, they change to the form as they occur n nature.
Question 14.
What does this tell us about the conditions under which iron articles rust?
Answer:
Corrosion of iron ( commonly known as rusting ) occurs in presence of moisture and air.
Page 250
Question 15.
What is the role of furnace in metallurgy?
Answer:
A furnace is used for heating the ores and crude metals to required temperatures in metallurgical operations.
Question 16.
How they bear large amounts of heat?
Answer:
The furnaces are lined inside with refractory materials and hence they can bear large amounts of heat.
The substances which are capable of withstanding very high temperatures without melting or becoming soft are called refractory materials.
![]()
Question 17.
Do all furnaces have same structure?
Answer:
No, all furnaces do not have same structure.
TS 10th Class Physical Science Principles of Metallurgy Activities
Activity 1
Question 1.
How do you classify ores based on their formula?
1) Look at the following ores.
2) Identify the metal present in each ore.
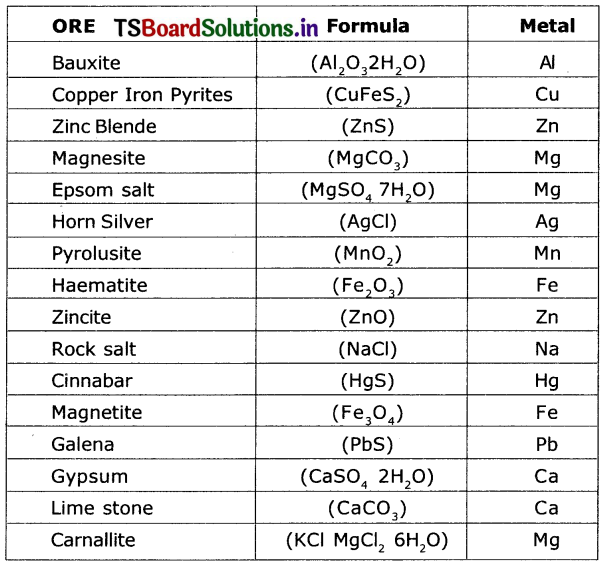
Now classify them as shown in the table.
Answer:
Classification of ores as oxides, sulphides, chlorides, carbonates, and sulphates is done as follows.
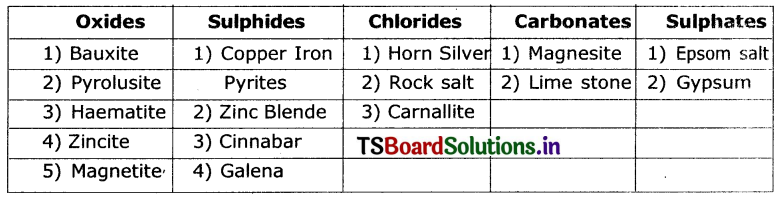
Activity 2
Question 2.
Show that both air and water are necessary for corrosion of iron.
Answer:
- Take three test tubes and place clean iron nails in each of them.
- Label these test tubes as A, B and C. Pour cork it.
- Pour boiled distilled water in test tube. B Add about 1 ml of oil and cork it. The oil will float on water and prevent the air from dissolving in the water.
- Put some anhydrous calcium chloride in test tube C and cork it. Anhydrous calcium chloride will absorb the moisture if any from the air. Leave these test tubes for a few days and then observe.
Observation: Iron nails rust in test tube A but they do not rust in test tubes B and C. In the test tube A the nails are exposed to both air and water. In the test tube B the nails are exposed to distilled water and the nails in test tube C are exposed to dry air only.
Inference: From this we can conclude that both air and water are necessary for corrosion of iron.